Comprehensive Genomic Identification and Expression Analysis of the Phosphate Transporter (PHT) Gene Family in Apple
- PMID: 28424713
- PMCID: PMC5371654
- DOI: 10.3389/fpls.2017.00426
Comprehensive Genomic Identification and Expression Analysis of the Phosphate Transporter (PHT) Gene Family in Apple
Abstract
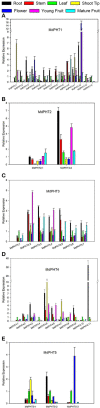
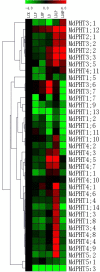
Elemental phosphorus (Pi) is essential to plant growth and development. The family of phosphate transporters (PHTs) mediates the uptake and translocation of Pi inside the plants. Members include five sub-cellular phosphate transporters that play different roles in Pi uptake and transport. We searched the Genome Database for Rosaceae and identified five clusters of phosphate transporters in apple (Malus domestica), including 37 putative genes. The MdPHT1 family contains 14 genes while MdPHT2 has two, MdPHT3 has seven, MdPHT4 has 11, and MdPHT5 has three. Our overview of this gene family focused on structure, chromosomal distribution and localization, phylogenies, and motifs. These genes displayed differential expression patterns in various tissues. For example, expression was high for MdPHT1;12, MdPHT3;6, and MdPHT3;7 in the roots, and was also increased in response to low-phosphorus conditions. In contrast, MdPHT4;1, MdPHT4;4, and MdPHT4;10 were expressed only in the leaves while transcript levels of MdPHT1;4, MdPHT1;12, and MdPHT5;3 were highest in flowers. In general, these 37 genes were regulated significantly in either roots or leaves in response to the imposition of phosphorus and/or drought stress. The results suggest that members of the PHT family function in plant adaptations to adverse growing environments. Our study will lay a foundation for better understanding the PHT family evolution and exploring genes of interest for genetic improvement in apple.
Keywords: apple; drought; expression; gene family; phosphate transporters; phosphorus stress.
Figures





Similar articles
-
Genome-Wide Analysis, Identification, and Transcriptional Profile of the Response to Abiotic Stress of the Purple Acid Phosphatases (PAP) Gene Family in Apple.Int J Mol Sci. 2025 Jan 24;26(3):1011. doi: 10.3390/ijms26031011. Int J Mol Sci. 2025. PMID: 39940779 Free PMC article.
-
Genome-Wide Identification, Expression Profiling, and Evolution of Phosphate Transporter Gene Family in Green Algae.Front Genet. 2020 Oct 8;11:590947. doi: 10.3389/fgene.2020.590947. eCollection 2020. Front Genet. 2020. PMID: 33133172 Free PMC article.
-
Characterizing the wheat (Triticum aestivum L.) phosphate transporter gene family and analyzing expression patterns in response to low phosphorus stress during the seedling stage.Front Plant Sci. 2025 Mar 27;16:1531642. doi: 10.3389/fpls.2025.1531642. eCollection 2025. Front Plant Sci. 2025. PMID: 40212870 Free PMC article.
-
Roles of plastid-located phosphate transporters in carotenoid accumulation.Front Plant Sci. 2022 Dec 15;13:1059536. doi: 10.3389/fpls.2022.1059536. eCollection 2022. Front Plant Sci. 2022. PMID: 36589064 Free PMC article. Review.
-
Roles, Regulation, and Agricultural Application of Plant Phosphate Transporters.Front Plant Sci. 2017 May 18;8:817. doi: 10.3389/fpls.2017.00817. eCollection 2017. Front Plant Sci. 2017. PMID: 28572810 Free PMC article. Review.
Cited by
-
Comprehensive sequence and expression profile analysis of the phosphate transporter gene family in soybean.Sci Rep. 2022 Dec 3;12(1):20883. doi: 10.1038/s41598-022-25378-w. Sci Rep. 2022. PMID: 36463363 Free PMC article.
-
Genome-Wide Identification and Expression Analysis of the Phosphate Transporter Gene Family in Zea mays Under Phosphorus Stress.Int J Mol Sci. 2025 Feb 9;26(4):1445. doi: 10.3390/ijms26041445. Int J Mol Sci. 2025. PMID: 40003911 Free PMC article.
-
Genome-Wide Identification and Analysis of Apple NITRATE TRANSPORTER 1/PEPTIDE TRANSPORTER Family (NPF) Genes Reveals MdNPF6.5 Confers High Capacity for Nitrogen Uptake under Low-Nitrogen Conditions.Int J Mol Sci. 2018 Sep 14;19(9):2761. doi: 10.3390/ijms19092761. Int J Mol Sci. 2018. PMID: 30223432 Free PMC article.
-
Genome-Wide Identification and Characterization of the PHT1 Gene Family and Its Response to Mycorrhizal Symbiosis in Salvia miltiorrhiza under Phosphate Stress.Genes (Basel). 2024 May 6;15(5):589. doi: 10.3390/genes15050589. Genes (Basel). 2024. PMID: 38790218 Free PMC article.
-
Molecular identification of the phosphate transporter family 1 (PHT1) genes and their expression profiles in response to phosphorus deprivation and other abiotic stresses in Brassica napus.PLoS One. 2019 Jul 25;14(7):e0220374. doi: 10.1371/journal.pone.0220374. eCollection 2019. PLoS One. 2019. PMID: 31344115 Free PMC article.
References
-
- Bieleski R. L. (1973). Phosphate pools, phosphate transport, and phosphate availability. Plant Physiol. 24, 225–252. 10.1146/annurev.pp.24.060173.001301 - DOI
-
- Chang S., Puryear J., Cairney J. (1993). A simple and efficient method for isolating RNA from pine trees. Plant Mol. Biol. Rep. 11, 113–116. 10.1007/BF02670468 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

