Postembedding Decalcification of Mineralized Tissue Sections Preserves the Integrity of Implanted Biomaterials and Minimizes Number of Experimental Animals
- PMID: 28424781
- PMCID: PMC5382295
- DOI: 10.1155/2017/2023853
Postembedding Decalcification of Mineralized Tissue Sections Preserves the Integrity of Implanted Biomaterials and Minimizes Number of Experimental Animals
Abstract
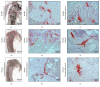
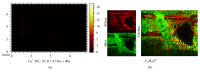
Bone histology of decalcified or undecalcified samples depends on the investigation. However, in research each method provides different information to answer the scientific question. Decalcification is the first step after sample fixation and governs what analysis is later feasible on the sections. Besides, decalcification is favored for immunostaining and in situ hybridization. Otherwise, sample decalcification can be damaging to bone biomaterials implants that contains calcium or strontium. On the other hand, after decalcification mineralization cannot be assessed using histology or imaging mass spectrometry. The current study provides a solution to the hardship caused by material presence within the bone tissue. The protocol presents a possibility of gaining sequential and alternating decalcified and undecalcified sections from the same bone sample. In this manner, investigations using histology, protein signaling, in situ hybridization, and mass spectrometry on the same sample can better answer the intended research question. Indeed, decalcification of sections and grindings resulted in well-preserved sample and biomaterials integrity. Immunostaining was comparable to that of classically decalcified samples. The study offers a novel approach that incites correlative analysis on the same sample and reduces the number of processed samples whether clinical biopsies or experimental animals.
Figures





Similar articles
-
Does decalcification alter the tissue sound speed of rabbit supraspinatus tendon insertion? In vitro measurement using scanning acoustic microscopy.Ultrasonics. 2006 Jul;44(3):297-301. doi: 10.1016/j.ultras.2006.03.002. Epub 2006 Apr 18. Ultrasonics. 2006. PMID: 16677677
-
Vitamin K2, a gamma-carboxylating factor of gla-proteins, normalizes the bone crystal nucleation impaired by Mg-insufficiency.Histol Histopathol. 2008 Nov;23(11):1353-66. doi: 10.14670/HH-23.1353. Histol Histopathol. 2008. PMID: 18785118
-
Application of ultrasound accelerates the decalcification process of bone matrix without affecting histological and immunohistochemical analysis.J Orthop Translat. 2018 Sep 5;17:112-120. doi: 10.1016/j.jot.2018.08.001. eCollection 2019 Apr. J Orthop Translat. 2018. PMID: 31194084 Free PMC article.
-
A comparative analysis of microscopic alterations in modern and ancient undecalcified and decalcified dry bones.Am J Phys Anthropol. 2018 Feb;165(2):363-369. doi: 10.1002/ajpa.23348. Epub 2017 Oct 27. Am J Phys Anthropol. 2018. PMID: 29076527
-
Histological, Histomorphometrical, and Biomechanical Studies of Bone-Implanted Medical Devices: Hard Resin Embedding.Biomed Res Int. 2020 Jan 17;2020:1804630. doi: 10.1155/2020/1804630. eCollection 2020. Biomed Res Int. 2020. PMID: 32420323 Free PMC article. Review.
Cited by
-
Landscape of Well-Coordinated Fracture Healing in a Mouse Model Using Molecular and Cellular Analysis.Int J Mol Sci. 2023 Feb 10;24(4):3569. doi: 10.3390/ijms24043569. Int J Mol Sci. 2023. PMID: 36834981 Free PMC article.
-
Enhancing Histological Techniques for Small Crustaceans: Evaluation of Fixation, Decalcification, and Enzymatic Digestion in Neocaridina Shrimp.Animals (Basel). 2025 Jun 10;15(12):1715. doi: 10.3390/ani15121715. Animals (Basel). 2025. PMID: 40564267 Free PMC article.
-
Confirmation of Calcium Phosphate Cement Biodegradation after Jawbone Augmentation around Dental Implants Using Three-Dimensional Visualization and Segmentation Software.Materials (Basel). 2021 Nov 22;14(22):7084. doi: 10.3390/ma14227084. Materials (Basel). 2021. PMID: 34832488 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

