A phase I dose-escalation study of Selumetinib in combination with Erlotinib or Temsirolimus in patients with advanced solid tumors
- PMID: 28424891
- PMCID: PMC5613062
- DOI: 10.1007/s10637-017-0459-7
A phase I dose-escalation study of Selumetinib in combination with Erlotinib or Temsirolimus in patients with advanced solid tumors
Erratum in
-
Erratum to: A phase I dose-escalation study of Selumetinib in combination with Erlotinib or Temsirolimus in patients with advanced solid tumors.Invest New Drugs. 2017 Oct;35(5):669. doi: 10.1007/s10637-017-0479-3. Invest New Drugs. 2017. PMID: 28676972 Free PMC article. No abstract available.
Abstract
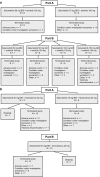
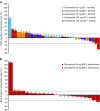
Background Combinations of molecularly targeted agents may provide optimal anti-tumor activity and improve clinical outcomes for patients with advanced cancers. Selumetinib (AZD6244, ARRY-142886) is an oral, potent and highly selective, allosteric inhibitor of MEK1/2, a component of the RAS/RAF/MEK/ERK pathway which is constitutively activated in many cancers. We investigated the safety, tolerability, and pharmacokinetics (PK) of selumetinib in combination with molecularly targeted drugs erlotinib or temsirolimus in patients with advanced solid tumors. Methods Two-part study: dose escalation, to determine the maximum tolerated dose (MTD) of selumetinib in combination with erlotinib 100 mg once daily (QD) or temsirolimus 25 mg once weekly, followed by dose expansion at the respective combination MTDs to further investigate safety and anti-tumor effects. Results 48 patients received selumetinib plus erlotinib and 32 patients received selumetinib plus temsirolimus. The MTD with erlotinib 100 mg QD was selumetinib 100 mg QD, with diarrhea being dose limiting. The most common all grade adverse events (AEs): diarrhea, rash, nausea, and fatigue. Four (8.3%) patients had ≥12 weeks stable disease. The MTD with temsirolimus 25 mg once weekly was selumetinib 50 mg twice daily (BID), with mucositis and neutropenia being dose limiting. The most commonly reported AEs: nausea, fatigue, diarrhea, and mucositis. Ten (31.3%) patients had ≥12 weeks stable disease. The combination PK profiles were comparable to previously observed monotherapy profiles. Conclusions MTDs were established for selumetinib in combination with erlotinib or temsirolimus. Overlapping toxicities prevented the escalation of selumetinib to its recommended phase II monotherapy dose of 75 mg BID.
Trial registration: ClinicalTrials.gov NCT00600496; registered 8 July 2009.
Keywords: Advanced solid tumors; Dose-escalation; Erlotinib; Selumetinib; Temsirolimus.
Conflict of interest statement
Author JI declares that he has no conflict of interest. Author RC declares that he has no conflict of interest. Author KK declares that he has no conflict of interest. Author HB declares that he has no conflict of interest. Author GC declares that at the time of the study he was an employee of AstraZeneca and held stock/share options. Author UE declares that he is an employee of AstraZeneca and holds stock/share options. Author HT declares that she is an employee of AstraZeneca and holds stock/share options. Author DC declares that she is a former employee of AstraZeneca and holds stock/share options. Author PL declares that she has no conflict of interest.
Figures
References
-
- Davies BR, Logie A, McKay JS, et al. AZD6244 (ARRY-142886), a potent inhibitor of mitogen-activated protein kinase/extracellular signal-regulated kinase kinase 1/2 kinases: mechanism of action in vivo, pharmacokinetic/pharmacodynamic relationship, and potential for combination in preclinical models. Mol Cancer Ther. 2007;6(8):2209–2019. doi: 10.1158/1535-7163.MCT-07-0231. - DOI - PubMed
-
- NCCN. NCCN Practice Guidelines in Oncology Melanoma (version 4.2014) (2014) http://www.nccn.org/professionals/physician_gls/pdf/melanoma.pdf. Accessed 18 August 2015
-
- Banerji U, Camidge DR, Verheul HM, et al. The first-in-human study of the hydrogen sulfate (Hyd-sulfate) capsule of the MEK1/2 inhibitor AZD6244 (ARRY-142886): a phase I open-label multicenter trial in patients with advanced cancer. Clin Cancer Res. 2010;16(5):1613–1623. doi: 10.1158/1078-0432.CCR-09-2483. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

