Overcoming chemotherapy drug resistance by targeting inhibitors of apoptosis proteins (IAPs)
- PMID: 28424988
- PMCID: PMC5486846
- DOI: 10.1007/s10495-017-1375-1
Overcoming chemotherapy drug resistance by targeting inhibitors of apoptosis proteins (IAPs)
Abstract
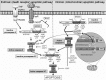
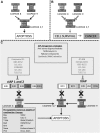
Inhibitors of apoptosis (IAPs) are a family of proteins that play a significant role in the control of programmed cell death (PCD). PCD is essential to maintain healthy cell turnover within tissue but also to fight disease or infection. Uninhibited, IAPs can suppress apoptosis and promote cell cycle progression. Therefore, it is unsurprising that cancer cells demonstrate significantly elevated expression levels of IAPs, resulting in improved cell survival, enhanced tumor growth and subsequent metastasis. Therapies to target IAPs in cancer has garnered substantial scientific interest and as resistance to anti-cancer agents becomes more prevalent, targeting IAPs has become an increasingly attractive strategy to re-sensitize cancer cells to chemotherapies, antibody based-therapies and TRAIL therapy. Antagonism strategies to modulate the actions of XIAP, cIAP1/2 and survivin are the central focus of current research and this review highlights advances within this field with particular emphasis upon the development and specificity of second mitochondria-derived activator of caspase (SMAC) mimetics (synthetic analogs of endogenously expressed inhibitors of IAPs SMAC/DIABLO). While we highlight the potential of SMAC mimetics as effective single agent or combinatory therapies to treat cancer we also discuss the likely clinical implications of resistance to SMAC mimetic therapy, occasionally observed in cancer cell lines.
Keywords: Chemotherapy resistance; Combination therapy; Extrinsic apoptotic pathway; Inhibitors of apoptosis proteins; Intrinsic apoptotic pathway.
Figures
Similar articles
-
Smac mimetics in combination with TRAIL selectively target cancer stem cells in nasopharyngeal carcinoma.Mol Cancer Ther. 2013 Sep;12(9):1728-37. doi: 10.1158/1535-7163.MCT-13-0017. Epub 2013 May 22. Mol Cancer Ther. 2013. PMID: 23699656
-
A Smac-mimetic sensitizes prostate cancer cells to TRAIL-induced apoptosis via modulating both IAPs and NF-kappaB.BMC Cancer. 2009 Nov 6;9:392. doi: 10.1186/1471-2407-9-392. BMC Cancer. 2009. PMID: 19895686 Free PMC article.
-
A small molecule Smac-mimic compound induces apoptosis and sensitizes TRAIL- and etoposide-induced apoptosis in breast cancer cells.Oncogene. 2005 Nov 10;24(49):7381-8. doi: 10.1038/sj.onc.1208888. Oncogene. 2005. PMID: 16044155
-
Therapeutic Small Molecules Target Inhibitor of Apoptosis Proteins in Cancers with Deregulation of Extrinsic and Intrinsic Cell Death Pathways.Clin Cancer Res. 2017 Mar 15;23(6):1379-1387. doi: 10.1158/1078-0432.CCR-16-2172. Epub 2016 Dec 30. Clin Cancer Res. 2017. PMID: 28039268 Free PMC article. Review.
-
Role of genomics-based strategies in overcoming chemotherapeutic resistance.Curr Pharm Biotechnol. 2004 Oct;5(5):471-80. doi: 10.2174/1389201043376698. Curr Pharm Biotechnol. 2004. PMID: 15544495 Review.
Cited by
-
[Effect of stable overexpression of XAF1 gene on biological characteristics of ovarian cancer A2780 cells].Nan Fang Yi Ke Da Xue Xue Bao. 2021 May 20;41(5):760-766. doi: 10.12122/j.issn.1673-4254.2021.05.18. Nan Fang Yi Ke Da Xue Xue Bao. 2021. PMID: 34134965 Free PMC article. Chinese.
-
Proteolysis-Targeting Chimeras (PROTACs) in Cancer Therapy: Present and Future.Molecules. 2022 Dec 12;27(24):8828. doi: 10.3390/molecules27248828. Molecules. 2022. PMID: 36557960 Free PMC article. Review.
-
Phytol and Heptacosane Are Possible Tools to Overcome Multidrug Resistance in an In Vitro Model of Acute Myeloid Leukemia.Pharmaceuticals (Basel). 2022 Mar 15;15(3):356. doi: 10.3390/ph15030356. Pharmaceuticals (Basel). 2022. PMID: 35337153 Free PMC article.
-
Pharmaceutical Reactivation of Attenuated Apoptotic Pathways Leads to Elimination of Osimertinib Drug-Tolerant Cells.Cancer Res Commun. 2022 Oct 31;2(10):1312-1325. doi: 10.1158/2767-9764.CRC-22-0066. eCollection 2022 Oct. Cancer Res Commun. 2022. PMID: 36969743 Free PMC article.
-
Anti-proliferative effects of the combination of Sulfamethoxazole and Quercetin via caspase3 and NFkB gene regulation: an in vitro and in vivo study.Naunyn Schmiedebergs Arch Pharmacol. 2022 Feb;395(2):227-246. doi: 10.1007/s00210-021-02174-3. Epub 2022 Jan 7. Naunyn Schmiedebergs Arch Pharmacol. 2022. PMID: 34994822
References
-
- Senan S, Brade A, Wang LH, Vansteenkiste J, Dakhil S, Biesma B, Martinez Aguillo M, Aerts J, Govindan R, Rubio-Viqueira B, Lewanski C, Gandara D, Choy H, Mok T, Hossain A, Iscoe N, Treat J, Koustenis A, San Antonio B, Chouaki N, Vokes E. PROCLAIM: randomized phase III trial of pemetrexed-cisplatin or etoposide-cisplatin plus thoracic radiation therapy followed by consolidation chemotherapy in locally advanced nonsquamous non-small-cell lung cancer. J Clin Oncol. 2016;34(9):953–962. doi: 10.1200/JCO.2015.64.8824. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

