Seizure frequency correlates with loss of dentate gyrus GABAergic neurons in a mouse model of temporal lobe epilepsy
- PMID: 28425097
- PMCID: PMC5963263
- DOI: 10.1002/cne.24226
Seizure frequency correlates with loss of dentate gyrus GABAergic neurons in a mouse model of temporal lobe epilepsy
Abstract
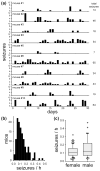
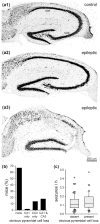
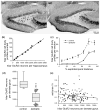
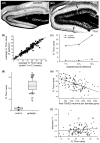
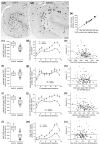
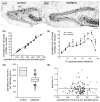
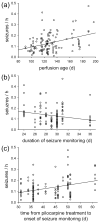
Epilepsy occurs in one of 26 people. Temporal lobe epilepsy is common and can be difficult to treat effectively. It can develop after brain injuries that damage the hippocampus. Multiple pathophysiological mechanisms involving the hippocampal dentate gyrus have been proposed. This study evaluated a mouse model of temporal lobe epilepsy to test which pathological changes in the dentate gyrus correlate with seizure frequency and help prioritize potential mechanisms for further study. FVB mice (n = 127) that had experienced status epilepticus after systemic treatment with pilocarpine 31-61 days earlier were video-monitored for spontaneous, convulsive seizures 9 hr/day every day for 24-36 days. Over 4,060 seizures were observed. Seizure frequency ranged from an average of one every 3.6 days to one every 2.1 hr. Hippocampal sections were processed for Nissl stain, Prox1-immunocytochemistry, GluR2-immunocytochemistry, Timm stain, glial fibrillary acidic protein-immunocytochemistry, glutamic acid decarboxylase in situ hybridization, and parvalbumin-immunocytochemistry. Stereological methods were used to measure hilar ectopic granule cells, mossy cells, mossy fiber sprouting, astrogliosis, and GABAergic interneurons. Seizure frequency was not significantly correlated with the generation of hilar ectopic granule cells, the number of mossy cells, the extent of mossy fiber sprouting, the extent of astrogliosis, or the number of GABAergic interneurons in the molecular layer or hilus. Seizure frequency significantly correlated with the loss of GABAergic interneurons in or adjacent to the granule cell layer, but not with the loss of parvalbumin-positive interneurons. These findings prioritize the loss of granule cell layer interneurons for further testing as a potential cause of temporal lobe epilepsy.
Keywords: GFAP; Prox1; RRID: AB_10000344; RRID: AB_10013382; RRID: AB_10064230; RRID: AB_2247874; Timm stain; hippocampus; mossy cell; pilocarpine.
© 2017 Wiley Periodicals, Inc.
Conflict of interest statement
The authors have no conflicts of interest.
Figures


References
-
- Andrioli A, Alonso-Nanclares L, Arellano JI, DeFilipe J. Quantitative analysis of parvalbumin-immunoreactive cells in the human epileptic hippocampus. Neuroscience. 2007;149:131–143. - PubMed
-
- Austin JE, Buckmaster PS. Recurrent excitation of granule cells with basal dendrites and low interneuron density and inhibitory postsynaptic current frequency in the dentate gyrus of macaque monkeys. The Journal of Comparative Neurology. 2004;476:205–218. - PubMed
-
- Babb TL, Brown WJ, Pretorius J, Davenport C, Lieb JP, Crandall PH. Temporal lobe volumetric cell densities in temporal lobe epilepsy. Epilepsia. 1984;25:729–740. - PubMed
-
- Babb TL, Kupfer WR, Pretorius JK, Crandall PH, Levesque MF. Synaptic reorganization by mossy fibers in human epileptic fascia dentata. Neuroscience. 1991;42:351–363. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

