Pneumonia risk in asthma patients using inhaled corticosteroids: a quasi-cohort study
- PMID: 28425216
- PMCID: PMC5555854
- DOI: 10.1111/bcp.13295
Pneumonia risk in asthma patients using inhaled corticosteroids: a quasi-cohort study
Abstract
Aim: Studies have linked the use of inhaled corticosteroids (ICSs) to excess pneumonia risk in chronic obstructive pulmonary disease patients. The risk in asthma patients remains unclear. The objective of the present study was to examine the risk of pneumonia with ICSs in asthma patients aged 12-35 years.
Methods: We formed a cohort of asthma patients treated from 1990 to 2007 using Quebec health insurance databases. Subjects were considered currently exposed if they had had an ICS dispensed within the 60 days prior to their pneumonia index event or matched person-moment. Secondary analyses investigated the risk of pneumonia according to ICS dose and type. Rate ratios (RRs) and rate differences (RDs) were both estimated through a quasi-cohort approach.
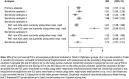
Results: The cohort included 152 412 subjects, of whom 1928 had a pneumonia event during follow-up. There was an increased risk of pneumonia associated with current use of ICSs [RR 1.83; 95% confidence interval (CI) 1.57, 2.14] or an excess risk of 1.44 cases per 1000 person-years (RD 1.44; 95% CI 1.03, 1.85). There was an excess pneumonia risk with low doses (RR 1.60; 95% CI 1.06, 2.45), moderate doses (RR 1.53; 95% CI 1.12, 2.08) and high doses (RR 1.96; 95% CI 1.64, 2.34) of ICSs, and with budesonide (RR 2.67; 95% CI 2.05, 3.49) and fluticasone (RR 1.93; 95% CI 1.58, 2.36), specifically relative to no use. When accounting for potential protopathic bias, the risk with current use of ICSs was attenuated (RR 1.48; 95% CI 1.22, 1.78).
Conclusion: ICS use in asthma patients appears to be associated with an increased risk of pneumonia and is present for both budesonide and fluticasone.
Keywords: adverse events; asthma; cohort study; inhaled corticosteroids; pneumonia; quasi-cohort.
© 2017 The British Pharmacological Society.
Figures

Similar articles
-
The airways microbiome of individuals with asthma treated with high and low doses of inhaled corticosteroids.PLoS One. 2020 Dec 30;15(12):e0244681. doi: 10.1371/journal.pone.0244681. eCollection 2020. PLoS One. 2020. PMID: 33378384 Free PMC article.
-
Risks of pneumonia in patients with asthma taking inhaled corticosteroids.Am J Respir Crit Care Med. 2011 Mar 1;183(5):589-95. doi: 10.1164/rccm.201005-0694OC. Epub 2010 Oct 1. Am J Respir Crit Care Med. 2011. PMID: 20889908
-
Comparison of Risk of Pneumonia Caused by Fluticasone Propionate versus Budesonide in Chronic Obstructive Pulmonary Disease: A Nationwide Retrospective Cohort Study.Int J Chron Obstruct Pulmon Dis. 2021 Nov 25;16:3229-3237. doi: 10.2147/COPD.S332151. eCollection 2021. Int J Chron Obstruct Pulmon Dis. 2021. PMID: 34858023 Free PMC article.
-
Risk of new onset diabetes mellitus in patients with asthma or COPD taking inhaled corticosteroids.Respir Med. 2012 Nov;106(11):1487-93. doi: 10.1016/j.rmed.2012.07.011. Epub 2012 Aug 15. Respir Med. 2012. PMID: 22902134 Review.
-
Intraclass Difference in Pneumonia Risk with Fluticasone and Budesonide in COPD: A Systematic Review of Evidence from Direct-Comparison Studies.Int J Chron Obstruct Pulmon Dis. 2020 Nov 11;15:2889-2900. doi: 10.2147/COPD.S269637. eCollection 2020. Int J Chron Obstruct Pulmon Dis. 2020. PMID: 33204085 Free PMC article.
Cited by
-
Unremitting Asthma as a Presentation of Pulmonary Nocardiosis: A Case Report.Cureus. 2024 Feb 22;16(2):e54722. doi: 10.7759/cureus.54722. eCollection 2024 Feb. Cureus. 2024. PMID: 38524073 Free PMC article.
-
The airways microbiome of individuals with asthma treated with high and low doses of inhaled corticosteroids.PLoS One. 2020 Dec 30;15(12):e0244681. doi: 10.1371/journal.pone.0244681. eCollection 2020. PLoS One. 2020. PMID: 33378384 Free PMC article.
-
Dilemma of Asthma Treatment in Mild Patients.Tuberc Respir Dis (Seoul). 2019 Jul;82(3):190-193. doi: 10.4046/trd.2018.0013. Epub 2018 Sep 28. Tuberc Respir Dis (Seoul). 2019. PMID: 30302951 Free PMC article. Review.
-
Use of inhaled corticosteroids and the risk of hospitalisation for pneumonia in children with asthma: a nationwide cohort study.Thorax. 2024 Apr 15;79(5):395-402. doi: 10.1136/thorax-2023-220742. Thorax. 2024. PMID: 38184370 Free PMC article.
-
Risk for pneumonia requiring hospitalization or emergency room visit according to delivery device for inhaled corticosteroid/long-acting beta-agonist in patients with chronic airway diseases as real-world evidence.Sci Rep. 2019 Aug 19;9(1):12004. doi: 10.1038/s41598-019-48355-2. Sci Rep. 2019. PMID: 31427602 Free PMC article.
References
-
- Chapman KR. The impact of budesonide and other inhaled corticosteroid therapies in the management of asthma in children and adults. Clin Ther 2003; 25 (Suppl. C): C2–14. - PubMed
-
- Lipworth BJ. Systemic adverse effects of inhaled corticosteroid therapy: a systematic review and meta‐analysis. Arch Intern Med 1999; 159: 941–955. - PubMed
-
- Ernst P, Suissa S. Systemic effects of inhaled corticosteroids. Curr Opin Pulm Med 2012; 18: 85–89. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

