FRET biosensor uncovers cAMP nano-domains at β-adrenergic targets that dictate precise tuning of cardiac contractility
- PMID: 28425435
- PMCID: PMC5411486
- DOI: 10.1038/ncomms15031
FRET biosensor uncovers cAMP nano-domains at β-adrenergic targets that dictate precise tuning of cardiac contractility
Abstract
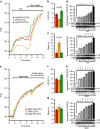
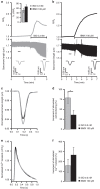
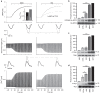
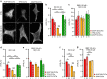
Compartmentalized cAMP/PKA signalling is now recognized as important for physiology and pathophysiology, yet a detailed understanding of the properties, regulation and function of local cAMP/PKA signals is lacking. Here we present a fluorescence resonance energy transfer (FRET)-based sensor, CUTie, which detects compartmentalized cAMP with unprecedented accuracy. CUTie, targeted to specific multiprotein complexes at discrete plasmalemmal, sarcoplasmic reticular and myofilament sites, reveals differential kinetics and amplitudes of localized cAMP signals. This nanoscopic heterogeneity of cAMP signals is necessary to optimize cardiac contractility upon adrenergic activation. At low adrenergic levels, and those mimicking heart failure, differential local cAMP responses are exacerbated, with near abolition of cAMP signalling at certain locations. This work provides tools and fundamental mechanistic insights into subcellular adrenergic signalling in normal and pathological cardiac function.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


Similar articles
-
Spatiotemporal dynamics of beta-adrenergic cAMP signals and L-type Ca2+ channel regulation in adult rat ventricular myocytes: role of phosphodiesterases.Circ Res. 2008 May 9;102(9):1091-100. doi: 10.1161/CIRCRESAHA.107.167817. Epub 2008 Mar 27. Circ Res. 2008. PMID: 18369156
-
Cyclic AMP imaging in adult cardiac myocytes reveals far-reaching beta1-adrenergic but locally confined beta2-adrenergic receptor-mediated signaling.Circ Res. 2006 Nov 10;99(10):1084-91. doi: 10.1161/01.RES.0000250046.69918.d5. Epub 2006 Oct 12. Circ Res. 2006. PMID: 17038640
-
A long lasting β1 adrenergic receptor stimulation of cAMP/protein kinase A (PKA) signal in cardiac myocytes.J Biol Chem. 2014 May 23;289(21):14771-81. doi: 10.1074/jbc.M113.542589. Epub 2014 Apr 8. J Biol Chem. 2014. PMID: 24713698 Free PMC article.
-
cAMP/PKA signaling compartmentalization in cardiomyocytes: Lessons from FRET-based biosensors.J Mol Cell Cardiol. 2019 Jun;131:112-121. doi: 10.1016/j.yjmcc.2019.04.020. Epub 2019 Apr 24. J Mol Cell Cardiol. 2019. PMID: 31028775 Review.
-
Investigating cardiac β-adrenergic nuclear signaling with FRET-based biosensors.Ann Endocrinol (Paris). 2021 Jun;82(3-4):198-200. doi: 10.1016/j.ando.2020.04.001. Epub 2020 Apr 7. Ann Endocrinol (Paris). 2021. PMID: 32482343 Review.
Cited by
-
Inhibition of adenylyl cyclase 1 by ST034307 inhibits IP3-evoked changes in sino-atrial node beat rate.Front Pharmacol. 2022 Aug 29;13:951897. doi: 10.3389/fphar.2022.951897. eCollection 2022. Front Pharmacol. 2022. PMID: 36105228 Free PMC article.
-
Impact of phosphodiesterases PDE3 and PDE4 on 5-hydroxytryptamine receptor4-mediated increase of cAMP in human atrial fibrillation.Naunyn Schmiedebergs Arch Pharmacol. 2021 Feb;394(2):291-298. doi: 10.1007/s00210-020-01968-1. Epub 2020 Sep 19. Naunyn Schmiedebergs Arch Pharmacol. 2021. PMID: 32949251 Free PMC article.
-
Regulation of cardiac function by cAMP nanodomains.Biosci Rep. 2023 Feb 27;43(2):BSR20220953. doi: 10.1042/BSR20220953. Biosci Rep. 2023. PMID: 36749130 Free PMC article. Review.
-
Cues to Opening Mechanisms From in Silico Electric Field Excitation of Cx26 Hemichannel and in Vitro Mutagenesis Studies in HeLa Transfectans.Front Mol Neurosci. 2018 May 31;11:170. doi: 10.3389/fnmol.2018.00170. eCollection 2018. Front Mol Neurosci. 2018. PMID: 29904340 Free PMC article.
-
cAMP Buffering via Liquid-Liquid Phase Separation.Function (Oxf). 2020 Dec 26;2(1):zqaa048. doi: 10.1093/function/zqaa048. eCollection 2021. Function (Oxf). 2020. PMID: 33511352 Free PMC article. No abstract available.
References
-
- Bers D. M. Cardiac excitation-contraction coupling. Nature 415, 198–205 (2002). - PubMed
-
- Zaccolo M. & Pozzan T. Discrete microdomains with high concentration of cAMP in stimulated rat neonatal cardiac myocytes. Science 295, 1711–1715 (2002). - PubMed
-
- Di Benedetto G. et al.. Protein kinase A type I and type II define distinct intracellular signaling compartments. Circ. Res. 103, 836–844 (2008). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL138539/HL/NHLBI NIH HHS/United States
- RG/12/18/30088/BHF_/British Heart Foundation/United Kingdom
- P01 HL080101/HL/NHLBI NIH HHS/United States
- R01 HL092097/HL/NHLBI NIH HHS/United States
- R01 HL131517/HL/NHLBI NIH HHS/United States
- R01 HL030077/HL/NHLBI NIH HHS/United States
- PG/15/5/31110/BHF_/British Heart Foundation/United Kingdom
- MR/L006855/1/MRC_/Medical Research Council/United Kingdom
- R01 HL105242/HL/NHLBI NIH HHS/United States
- PG/10/75/28537/BHF_/British Heart Foundation/United Kingdom
- R01 HL126802/HL/NHLBI NIH HHS/United States
- R37 HL030077/HL/NHLBI NIH HHS/United States
- RG/12/3/29423/BHF_/British Heart Foundation/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources

