Oncogenic activity of SOX1 in glioblastoma
- PMID: 28425506
- PMCID: PMC5397861
- DOI: 10.1038/srep46575
Oncogenic activity of SOX1 in glioblastoma
Abstract
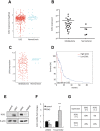
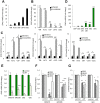
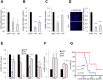
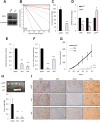
Glioblastoma remains the most common and deadliest type of brain tumor and contains a population of self-renewing, highly tumorigenic glioma stem cells (GSCs), which contributes to tumor initiation and treatment resistance. Developmental programs participating in tissue development and homeostasis re-emerge in GSCs, supporting the development and progression of glioblastoma. SOX1 plays an important role in neural development and neural progenitor pool maintenance. Its impact on glioblastoma remains largely unknown. In this study, we have found that high levels of SOX1 observed in a subset of patients correlate with lower overall survival. At the cellular level, SOX1 expression is elevated in patient-derived GSCs and it is also higher in oncosphere culture compared to differentiation conditions in conventional glioblastoma cell lines. Moreover, genetic inhibition of SOX1 in patient-derived GSCs and conventional cell lines decreases self-renewal and proliferative capacity in vitro and tumor initiation and growth in vivo. Contrarily, SOX1 over-expression moderately promotes self-renewal and proliferation in GSCs. These functions seem to be independent of its activity as Wnt/β-catenin signaling regulator. In summary, these results identify a functional role for SOX1 in regulating glioma cell heterogeneity and plasticity, and suggest SOX1 as a potential target in the GSC population in glioblastoma.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

References
-
- Phillips H. S. et al.. Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell 9, 157–173 (2006). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

