Bone Marrow, Adipose, and Lung Tissue-Derived Murine Mesenchymal Stromal Cells Release Different Mediators and Differentially Affect Airway and Lung Parenchyma in Experimental Asthma
- PMID: 28425576
- PMCID: PMC5689762
- DOI: 10.1002/sctm.16-0398
Bone Marrow, Adipose, and Lung Tissue-Derived Murine Mesenchymal Stromal Cells Release Different Mediators and Differentially Affect Airway and Lung Parenchyma in Experimental Asthma
Abstract
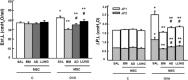
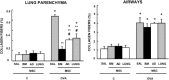
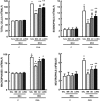
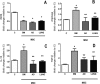
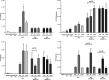
Mesenchymal stromal cells (MSCs) from different sources have differential effects on lung injury. To compare the effects of murine MSCs from bone marrow (BM), adipose tissue (AD), and lung tissue (LUNG) on inflammatory and remodeling processes in experimental allergic asthma, female C57BL/6 mice were sensitized and challenged with ovalbumin (OVA) or saline (C). Twenty-four hours after the last challenge, mice received either saline (50 µl, SAL), BM-MSCs, AD-MSCs, or LUNG-MSCs (105 cells per mouse in 50 µl total volume) intratracheally. At 1 week, BM-MSCs produced significantly greater reductions in resistive and viscoelastic pressures, bronchoconstriction index, collagen fiber content in lung parenchyma (but not airways), eosinophil infiltration, and levels of interleukin (IL)-4, IL-13, transforming growth factor (TGF)-β, and vascular endothelial growth factor (VEGF) in lung homogenates compared to AD-MSCs and LUNG-MSCs. Only BM-MSCs increased IL-10 and interferon (IFN)-γ in lung tissue. In parallel in vitro experiments, BM-MSCs increased M2 macrophage polarization, whereas AD-MSCs and LUNG-MSCs had higher baseline levels of IL-4, insulin-like growth factor (IGF), and VEGF secretion. Exposure of MSCs to serum specimens obtained from asthmatic mice promoted reductions in secretion of these mediators, particularly in BM-MSCs. Intratracheally administered BM-MSCs, AD-MSCs, and LUNG-MSCs were differentially effective at reducing airway inflammation and remodeling and improving lung function in the current model of allergic asthma. In conclusion, intratracheal administration of MSCs from BM, AD, and LUNG were differentially effective at reducing airway inflammation and remodeling and improving lung function comparably reduced inflammation and fibrogenesis in this asthma model. However, altered lung mechanics and lung remodeling responded better to BM-MSCs than to AD-MSCs or LUNG-MSCs. Moreover, each type of MSC was differentially affected in a surrogate in vitro model of the in vivo lung environment. Stem Cells Translational Medicine 2017;6:1557-1567.
Keywords: Asthma; Fibrosis; Inflammation; Macrophage; Mesenchymal stromal cells.
© 2017 The Authors Stem Cells Translational Medicine published by Wiley Periodicals, Inc. on behalf of AlphaMed Press.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

