Managing the sequence-specificity of antisense oligonucleotides in drug discovery
- PMID: 28426096
- PMCID: PMC5389529
- DOI: 10.1093/nar/gkx056
Managing the sequence-specificity of antisense oligonucleotides in drug discovery
Abstract
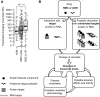
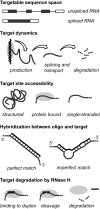
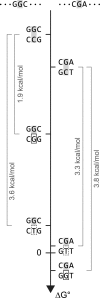
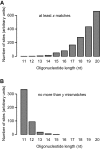
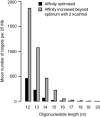
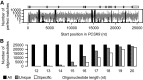
All drugs perturb the expression of many genes in the cells that are exposed to them. These gene expression changes can be divided into effects resulting from engaging the intended target and effects resulting from engaging unintended targets. For antisense oligonucleotides, developments in bioinformatics algorithms, and the quality of sequence databases, allow oligonucleotide sequences to be analyzed computationally, in terms of the predictability of their interactions with intended and unintended RNA targets. Applying these tools enables selection of sequence-specific oligonucleotides where no- or only few unintended RNA targets are expected. To evaluate oligonucleotide sequence-specificity experimentally, we recommend a transcriptomics protocol where two or more oligonucleotides targeting the same RNA molecule, but with entirely different sequences, are evaluated together. This helps to clarify which changes in cellular RNA levels result from downstream processes of engaging the intended target, and which are likely to be related to engaging unintended targets. As required for all classes of drugs, the toxic potential of oligonucleotides must be evaluated in cell- and animal models before clinical testing. Since potential adverse effects related to unintended targeting are sequence-dependent and therefore species-specific, in vitro toxicology assays in human cells are especially relevant in oligonucleotide drug discovery.
© The Author(s) 2017. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures



References
-
- Kakiuchi-Kiyota S., Koza-Taylor P.H., Mantena S.R., Nelms L.F., Enayetallah A.E., Hollingshead B.D., Burdick A.D., Reed L.A., Warneke J.A., Whiteley L.O. et al. . Comparison of hepatic transcription profiles of locked ribonucleic acid antisense oligonucleotides: evidence of distinct pathways contributing to non-target mediated toxicity in mice. Toxicol. Sci. 2014; 138:234–248. - PubMed
-
- Burel S.A., Hart C.E., Cauntay P., Hsiao J., Machemer T., Katz M., Watt A., Bui H.-H., Younis H., Sabripour M. et al. . Hepatotoxicity of high affinity gapmer antisense oligonucleotides is mediated by RNase H1 dependent promiscuous reduction of very long pre-mRNA transcripts. Nucleic Acids Res. 2015; 44:2093–2109. - PMC - PubMed
-
- Lamb J., Crawford E.D., Peck D., Modell J.W., Blat I.C., Wrobel M.J., Lerner J., Brunet J.-P., Subramanian A., Ross K.N. et al. . The Connectivity Map: using gene-expression signatures to connect small molecules, genes, and disease. Science. 2006; 313:1929–1935. - PubMed
-
- Lamb J. The Connectivity Map: a new tool for biomedical research. Nat. Rev. Cancer. 2007; 7:54–60. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

