Transient exposure to androgens induces a remarkable self-sustained quiescent state in dispersed prostate cancer cells
- PMID: 28426320
- PMCID: PMC5444360
- DOI: 10.1080/15384101.2017.1310345
Transient exposure to androgens induces a remarkable self-sustained quiescent state in dispersed prostate cancer cells
Abstract
Cellular quiescence is a reversible cell growth arrest that is often assumed to require a persistence of non-permissive external growth conditions for its maintenance. In this work, we showed that androgen could induce a quiescent state that is self-sustained in a cell-autonomous manner through a "hit and run" mechanism in androgen receptor-expressing prostate cancer cells. This phenomenon required the set-up of a sustained redox imbalance and TGFβ/BMP signaling that were dependent on culturing cells at low density. At medium cell density, androgens failed to induce such a self-sustained quiescent state, which correlated with a lesser induction of cell redox imbalance and oxidative stress markers like CDKN1A. These effects of androgens could be mimicked by transient overexpression of CDKN1A that triggered its own expression and a sustained SMAD phosphorylation in cells cultured at low cell density. Overall, our data suggest that self-sustained but fully reversible quiescent states might constitute a general response of dispersed cancer cells to stress conditions.
Keywords: Androgen; BMP; CDKN1A; cellular quiescence; feedback loops; oxidative stress; prostate cancer.
Figures
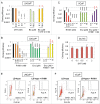
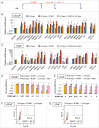
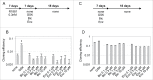


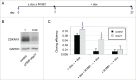
Comment in
-
Rationale for bipolar androgen therapy (BAT) for metastatic prostate cancer.Cell Cycle. 2017 Sep 17;16(18):1639-1640. doi: 10.1080/15384101.2017.1360645. Epub 2017 Aug 18. Cell Cycle. 2017. PMID: 28820291 Free PMC article. No abstract available.
Similar articles
-
SMAD signaling and redox imbalance cooperate to induce prostate cancer cell dormancy.Cell Cycle. 2015;14(8):1218-31. doi: 10.1080/15384101.2015.1014145. Cell Cycle. 2015. PMID: 25706341 Free PMC article.
-
Bone morphogenetic protein-2 induces hypophosphorylation of Rb protein and repression of E2F in androgen-treated LNCaP human prostate cancer cells.Int J Mol Med. 2005 Feb;15(2):253-8. Int J Mol Med. 2005. PMID: 15647840
-
BMP signals inhibit proliferation and in vivo tumor growth of androgen-insensitive prostate carcinoma cells.Oncogene. 2004 Dec 16;23(58):9326-35. doi: 10.1038/sj.onc.1208127. Oncogene. 2004. PMID: 15531927
-
Oxidative stress and androgen receptor signaling in the development and progression of castration-resistant prostate cancer.Free Radic Biol Med. 2011 Oct 1;51(7):1320-8. doi: 10.1016/j.freeradbiomed.2011.07.011. Epub 2011 Jul 23. Free Radic Biol Med. 2011. PMID: 21820046 Review.
-
Androgen action in the prostate gland.Minerva Urol Nefrol. 2012 Mar;64(1):35-49. Minerva Urol Nefrol. 2012. PMID: 22402316 Review.
Cited by
-
Discovery proteomics defines androgen-regulated glycoprotein networks in prostate cancer cells, as well as putative biomarkers of prostatic diseases.Sci Rep. 2021 Nov 15;11(1):22208. doi: 10.1038/s41598-021-01554-2. Sci Rep. 2021. PMID: 34782677 Free PMC article.
-
Mechanistic target of rapamycin (mTOR) regulates self-sustained quiescence, tumor indolence, and late clinical metastasis in a Beclin-1-dependent manner.Cell Cycle. 2023 Mar;22(5):542-564. doi: 10.1080/15384101.2022.2123187. Epub 2022 Sep 19. Cell Cycle. 2023. PMID: 36123968 Free PMC article.
-
Suppressing BCL-XL increased the high dose androgens therapeutic effect to better induce the Enzalutamide-resistant prostate cancer autophagic cell death.Cell Death Dis. 2021 Jan 11;12(1):68. doi: 10.1038/s41419-020-03321-z. Cell Death Dis. 2021. PMID: 33431795 Free PMC article.
-
Supraphysiologic Testosterone Therapy in the Treatment of Prostate Cancer: Models, Mechanisms and Questions.Cancers (Basel). 2017 Dec 6;9(12):166. doi: 10.3390/cancers9120166. Cancers (Basel). 2017. PMID: 29210989 Free PMC article. Review.
-
The testosterone paradox of advanced prostate cancer: mechanistic insights and clinical implications.Nat Rev Urol. 2023 May;20(5):265-278. doi: 10.1038/s41585-022-00686-y. Epub 2022 Dec 21. Nat Rev Urol. 2023. PMID: 36543976 Free PMC article. Review.
References
-
- Havard M, Dautry F, Tchenio T. A dormant state modulated by osmotic pressure controls clonogenicity of prostate cancer cells. J Biol Chem 2011; 286:44177-86; PMID:22039055; http://dx.doi.org/10.1074/jbc.M111.262709 - DOI - PMC - PubMed
-
- Bui AT, Laurent F, Havard M, Dautry F, Tchenio T. SMAD signaling and redox imbalance cooperate to induce prostate cancer cell dormancy. Cell Cycle 2015; 14:1218-31; PMID:25706341; http://dx.doi.org/10.1080/15384101.2015.1014145 - DOI - PMC - PubMed
-
- Wang ZA, Shen MM. Revisiting the concept of cancer stem cells in prostate cancer. Oncogene 2011; 30:1261-71; PMID:21119602; http://dx.doi.org/10.1038/onc.2010.530 - DOI - PubMed
-
- Choi N, Zhang B, Zhang L, Ittmann M, Xin L. Adult murine prostate basal and luminal cells are self-sustained lineages that can both serve as targets for prostate cancer initiation. Cancer Cell 2012; 21:253-65; PMID:22340597; http://dx.doi.org/10.1016/j.ccr.2012.01.005 - DOI - PMC - PubMed
-
- Wadosky KM, Koochekpour S. Molecular mechanisms underlying resistance to androgen deprivation therapy in prostate cancer. Oncotarget 2016; 7:64447-70; PMID:27487144; http://dx.doi.org/10.18632/oncotarget.10901 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
