Cheminformatics-aided discovery of small-molecule Protein-Protein Interaction (PPI) dual inhibitors of Tumor Necrosis Factor (TNF) and Receptor Activator of NF-κB Ligand (RANKL)
- PMID: 28426652
- PMCID: PMC5398486
- DOI: 10.1371/journal.pcbi.1005372
Cheminformatics-aided discovery of small-molecule Protein-Protein Interaction (PPI) dual inhibitors of Tumor Necrosis Factor (TNF) and Receptor Activator of NF-κB Ligand (RANKL)
Abstract
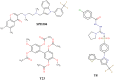
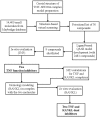
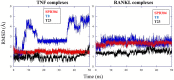
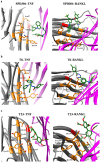
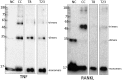
We present an in silico drug discovery pipeline developed and applied for the identification and virtual screening of small-molecule Protein-Protein Interaction (PPI) compounds that act as dual inhibitors of TNF and RANKL through the trimerization interface. The cheminformatics part of the pipeline was developed by combining structure-based with ligand-based modeling using the largest available set of known TNF inhibitors in the literature (2481 small molecules). To facilitate virtual screening, the consensus predictive model was made freely available at: http://enalos.insilicotox.com/TNFPubChem/. We thus generated a priority list of nine small molecules as candidates for direct TNF function inhibition. In vitro evaluation of these compounds led to the selection of two small molecules that act as potent direct inhibitors of TNF function, with IC50 values comparable to those of a previously-described direct inhibitor (SPD304), but with significantly reduced toxicity. These molecules were also identified as RANKL inhibitors and validated in vitro with respect to this second functionality. Direct binding of the two compounds was confirmed both for TNF and RANKL, as well as their ability to inhibit the biologically-active trimer forms. Molecular dynamics calculations were also carried out for the two small molecules in each protein to offer additional insight into the interactions that govern TNF and RANKL complex formation. To our knowledge, these compounds, namely T8 and T23, constitute the second and third published examples of dual small-molecule direct function inhibitors of TNF and RANKL, and could serve as lead compounds for the development of novel treatments for inflammatory and autoimmune diseases.
Conflict of interest statement
Georgia Melagraki, Georgios Leonis and Antreas Afantitis are employed by Novamechanics Ltd, a drug design company. Other authors declare that there are no conflicts of interest.
Figures


References
-
- Beutler B, Milsark IW, Cerami AC. Passive immunization against cachectin/tumor necrosis factor protects mice from lethal effect of endotoxin. Science. 1985;229: 869–871. - PubMed
-
- Kollias G, Douni E, Kassiotis G, Kontoyiannis D. On the role of tumor necrosis factor and receptors in models of multiorgan failure, rheumatoid arthritis, multiple sclerosis and inflammatory bowel disease. Immunol Rev. 1999;169: 175–194. - PubMed
-
- Black RA, Rauch CT, Kozlosky CJ, Peschon JJ, Slack JL, Wolfson MF, et al. A metalloproteinase disintegrin that releases tumour-necrosis factor-alpha from cells. Nature. 1997. pp. 729–733. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

