Bioenergetic status modulates motor neuron vulnerability and pathogenesis in a zebrafish model of spinal muscular atrophy
- PMID: 28426667
- PMCID: PMC5417717
- DOI: 10.1371/journal.pgen.1006744
Bioenergetic status modulates motor neuron vulnerability and pathogenesis in a zebrafish model of spinal muscular atrophy
Abstract
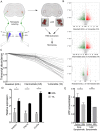
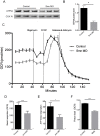
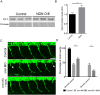
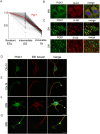
Degeneration and loss of lower motor neurons is the major pathological hallmark of spinal muscular atrophy (SMA), resulting from low levels of ubiquitously-expressed survival motor neuron (SMN) protein. One remarkable, yet unresolved, feature of SMA is that not all motor neurons are equally affected, with some populations displaying a robust resistance to the disease. Here, we demonstrate that selective vulnerability of distinct motor neuron pools arises from fundamental modifications to their basal molecular profiles. Comparative gene expression profiling of motor neurons innervating the extensor digitorum longus (disease-resistant), gastrocnemius (intermediate vulnerability), and tibialis anterior (vulnerable) muscles in mice revealed that disease susceptibility correlates strongly with a modified bioenergetic profile. Targeting of identified bioenergetic pathways by enhancing mitochondrial biogenesis rescued motor axon defects in SMA zebrafish. Moreover, targeting of a single bioenergetic protein, phosphoglycerate kinase 1 (Pgk1), was found to modulate motor neuron vulnerability in vivo. Knockdown of pgk1 alone was sufficient to partially mimic the SMA phenotype in wild-type zebrafish. Conversely, Pgk1 overexpression, or treatment with terazosin (an FDA-approved small molecule that binds and activates Pgk1), rescued motor axon phenotypes in SMA zebrafish. We conclude that global bioenergetics pathways can be therapeutically manipulated to ameliorate SMA motor neuron phenotypes in vivo.
Conflict of interest statement
THG is Chair of the Scientific and Clinical Advisory Group of the SMA Trust, and sits on advisory boards for the Association Française contre les Myopathies (AFM) and SMA Europe.
Figures


References
-
- Lefebvre S, Burglen L, Reboullet S, Clermont O, Burlet P, Viollet L, et al. Identification and characterization of a spinal muscular atrophy-determining gene. Cell. 1995;80(1):155–65. - PubMed
-
- Monani UR, Lorson CL, Parsons DW, Prior TW, Androphy EJ, Burghes AH, et al. A single nucleotide difference that alters splicing patterns distinguishes the SMA gene SMN1 from the copy gene SMN2. Hum Mol Genet. 1999;8(7):1177–83. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

