Vibrotactile stimulation: A non-pharmacological intervention for opioid-exposed newborns
- PMID: 28426726
- PMCID: PMC5398650
- DOI: 10.1371/journal.pone.0175981
Vibrotactile stimulation: A non-pharmacological intervention for opioid-exposed newborns
Abstract
Objective: To examine the therapeutic potential of stochastic vibrotactile stimulation (SVS) as a complementary non-pharmacological intervention for withdrawal in opioid-exposed newborns.
Study design: A prospective, within-subjects single-center study was conducted in 26 opioid-exposed newborns (>37 weeks; 16 male) hospitalized since birth and treated pharmacologically for Neonatal Abstinence Syndrome. A specially-constructed mattress delivered low-level SVS (30-60Hz, 10-12μm RMS), alternated in 30-min intervals between continuous vibration (ON) and no vibration (OFF) over a 6-8 hr session. Movement activity, heart rate, respiratory rate, axillary temperature and blood-oxygen saturation were calculated separately for ON and OFF.
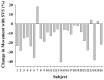
Results: There was a 35% reduction in movement activity with SVS (p<0.001), with significantly fewer movement periods >30 sec duration for ON than OFF (p = 0.003). Incidents of tachypneic breaths and tachycardic heart beats were each significantly reduced with SVS, whereas incidents of eupneic breaths and eucardic heart beats each significantly increased with SVS (p<0.03). Infants maintained body temperature and arterial-blood oxygen level independent of stimulation condition.
Conclusions: SVS reduced hyperirritability and pathophysiological instabilities commonly observed in pharmacologically-managed opioid-exposed newborns. SVS may provide an effective complementary therapeutic intervention for improving autonomic function in newborns with Neonatal Abstinence Syndrome.
Conflict of interest statement
Figures


References
-
- Substance Abuse and Mental Health Services Administration. Results from the 2009 National Survey on Drug Use and Health: Volume I. Summary of National Findings (Office of Applied Studies, NSDUH Series H-38A, HHS Publication No. SMA 10–4586 Findings). Rockville, MD, 2010. 2010 2010. Report No.
-
- Patrick SW, Davis MM, Lehman CU, Cooper WO. Increasing incidence and geographic distribution of neonatal abstinence syndrome: United States 2009 to 2012. J Perinatol. 2015;35(8):667. - PubMed
-
- Patrick SW, Schumacher RE, Benneyworth BD, Krans EE, McAllister JM, Davis MM. Neonatal abstinence syndrome and associated health care expenditures: United States, 2000–2009. JAMA. 2012;307(18):1934–40. doi: 10.1001/jama.2012.3951 - DOI - PubMed
-
- Tolia VN, Patrick SW, Bennett MM, Murthy K, Sousa J, Smith PB, et al. Increasing Incidence of the Neonatal Abstinence Syndrome in U.S. Neonatal ICUs. New England Journal of Medicine. 2015;372(22):2118–26. doi: 10.1056/NEJMsa1500439 - DOI - PubMed
-
- Barr GA, McPhie-Lalmansingh A, Perez J, Riley M. Changing mechanisms of opiate tolerance and withdrawal during early development: animal models of the human experience. ILAR J. 2011;52(3):329–41. doi: 10.1093/ilar.52.3.329 - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

