Dendritic cell maturation, but not type I interferon exposure, restricts infection by HTLV-1, and viral transmission to T-cells
- PMID: 28426803
- PMCID: PMC5413061
- DOI: 10.1371/journal.ppat.1006353
Dendritic cell maturation, but not type I interferon exposure, restricts infection by HTLV-1, and viral transmission to T-cells
Erratum in
-
Correction: Dendritic cell maturation, but not type I interferon exposure, restricts infection by HTLV-1, and viral transmission to T-cells.PLoS Pathog. 2017 Jul 11;13(7):e1006494. doi: 10.1371/journal.ppat.1006494. eCollection 2017 Jul. PLoS Pathog. 2017. PMID: 28700737 Free PMC article.
Abstract
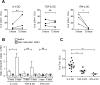
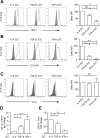
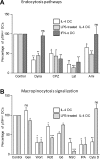
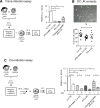
Human T lymphotropic Virus type 1 (HTLV-1) is the etiological agent of Adult T cell Leukemia/Lymphoma (ATLL) and HTLV-1-Associated Myelopathy/Tropical Spastic Paraparesis (HAM/TSP). Both CD4+ T-cells and dendritic cells (DCs) infected with HTLV-1 are found in peripheral blood from HTLV-1 carriers. We previously demonstrated that monocyte-derived IL-4 DCs are more susceptible to HTLV-1 infection than autologous primary T-cells, suggesting that DC infection precedes T-cell infection. However, during blood transmission, breast-feeding or sexual transmission, HTLV-1 may encounter different DC subsets present in the blood, the intestinal or genital mucosa respectively. These different contacts may impact HTLV-1 ability to infect DCs and its subsequent transfer to T-cells. Using in vitro monocyte-derived IL-4 DCs, TGF-β DCs and IFN-α DCs that mimic DCs contacting HTLV-1 in vivo, we show here that despite their increased ability to capture HTLV-1 virions, IFN-α DCs restrict HTLV-1 productive infection. Surprisingly, we then demonstrate that it is not due to the antiviral activity of type-I interferon produced by IFN-α DCs, but that it is likely to be linked to a distinct trafficking route of HTLV-1 in IL-4 DCs vs. IFN-α DCs. Finally, we demonstrate that, in contrast to IL-4 DCs, IFN-α DCs are impaired in their capacity to transfer HTLV-1 to CD4 T-cells, both after viral capture and trans-infection and after their productive infection. In conclusion, the nature of the DCs encountered by HTLV-1 upon primo-infection and the viral trafficking route through the vesicular pathway of these cells determine the efficiency of viral transmission to T-cells, which may condition the fate of infection.
Conflict of interest statement
The authors have declared that no competing of interests exit.
Figures





References
-
- Gessain A, Cassar O (2012) Epidemiological Aspects and World Distribution of HTLV-1 Infection. Front Microbiol 3: 388 doi: 10.3389/fmicb.2012.00388 - DOI - PMC - PubMed
-
- Bruhn R, Mahieux R, Murphy EL (In press) Human lymphotropic viruses: HTLV-1 and HTLV-2.: Clinical Virology 4th edition, ASM press.
-
- Willems L, Hasegawa H, Accolla R, Bangham C, Bazarbachi A, et al. (2016) Reducing the global burden of HTLV-1 infection: An agenda for research and action. Antiviral Res 137: 41–48. doi: 10.1016/j.antiviral.2016.10.015 - DOI - PubMed
-
- Uchiyama T, Yodoi J, Sagawa K, Takatsuki K, Uchino H (1977) Adult T-cell leukemia: clinical and hematologic features of 16 cases. Blood 50: 481–492. - PubMed
-
- Gessain A, Barin F, Vernant J, Gout O, Maurs L, et al. (1985) Antibodies to human T-lymphotropic virus type-I in patients with tropical spastic paraparesis. Lancet 2: 407–410. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

