The Therapeutic Efficacy of Botulinum Toxin in Treating Scleroderma-Associated Raynaud's Phenomenon: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial
- PMID: 28426903
- PMCID: PMC5529251
- DOI: 10.1002/art.40123
The Therapeutic Efficacy of Botulinum Toxin in Treating Scleroderma-Associated Raynaud's Phenomenon: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial
Abstract
Objective: To assess the therapeutic efficacy of local injections of botulinum toxin type A (Btx-A) in improving blood flow to the hands of patients with Raynaud's phenomenon (RP) secondary to scleroderma.
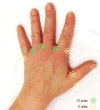
Methods: In this randomized, double-blind, placebo-controlled clinical trial, patients with scleroderma-associated RP received Btx-A (50 units in 2.5 ml sterile saline) in one randomly selected hand and sterile saline (2.5 ml) in the opposite hand. Follow-up at 1 and 4 months postinjection included laser Doppler imaging of hands, patient-reported outcomes, and physical examination. We compared outcomes using paired t-tests and population-average generalized models with generalized estimating equations.
Results: Of 40 patients enrolled, 25 had limited scleroderma and 15 had diffuse scleroderma. From baseline to 1-month follow-up, there was a greater reduction in average blood flow in Btx-A-treated hands compared to placebo-treated hands. The model estimated that this difference was statistically significant (average difference -30.08 flux units [95% confidence interval -56.19, -3.98], P for interaction = 0.024). This difference was mainly influenced by patients with longstanding RP and diffuse scleroderma. Change in blood flow at 4-month follow-up was not significantly different between groups. Clinical measures (QuickDASH, McCabe Cold Sensitivity Score, pain on a visual analog scale, and Raynaud's Condition Score) improved slightly for Btx-A-treated hands.
Conclusion: Our laboratory-based laser Doppler imaging flow data do not support using Btx-A to treat RP in all scleroderma patients. The secondary clinical outcomes suggest some positive effect, but its clinical meaningfulness is questionable. The role of Btx-A in treating RP should be further studied with more homogeneous patient populations and in unique clinical situations such as acute digital ischemia.
Trial registration: ClinicalTrials.gov NCT02165111 NCT02165111.
© 2017, American College of Rheumatology.
Figures




Similar articles
-
[Botulinum toxin type A contribution in the treatment of Raynaud's phenomenon due to systemic sclerosis].Ann Chir Plast Esthet. 2013 Dec;58(6):658-62. doi: 10.1016/j.anplas.2011.11.001. Epub 2011 Dec 26. Ann Chir Plast Esthet. 2013. PMID: 22204894 French.
-
A prospective study of the use of botulinum toxin injections in the treatment of Raynaud's syndrome associated with scleroderma.J Hand Surg Eur Vol. 2014 Oct;39(8):876-80. doi: 10.1177/1753193413516242. Epub 2013 Dec 24. J Hand Surg Eur Vol. 2014. PMID: 24369360
-
Botulinum Toxin A Treatment for Primary and Secondary Raynaud's Phenomenon in Teenagers.Dermatol Surg. 2021 Jan 1;47(1):61-64. doi: 10.1097/DSS.0000000000002397. Dermatol Surg. 2021. PMID: 32371783
-
Botulinum toxin A treatment of Raynaud's phenomenon: a review.Semin Arthritis Rheum. 2012 Feb;41(4):599-603. doi: 10.1016/j.semarthrit.2011.07.006. Epub 2011 Aug 24. Semin Arthritis Rheum. 2012. PMID: 21868066 Review.
-
Botulinum Toxin for the Treatment of Raynaud's Conditions of the Hand: Clinical Practice Updates and Future Directions.Toxins (Basel). 2024 Nov 1;16(11):472. doi: 10.3390/toxins16110472. Toxins (Basel). 2024. PMID: 39591227 Free PMC article. Review.
Cited by
-
Evaluation of Clinical Recovery After Surgical Treatment for Hand Ischemia From Vasospastic and Occlusive Disease Using PROMIS.Hand (N Y). 2023 Jan;18(1):15-21. doi: 10.1177/1558944721999727. Epub 2021 Mar 31. Hand (N Y). 2023. PMID: 33789521 Free PMC article.
-
New perspectives in the imaging of Raynaud's phenomenon.Eur J Rheumatol. 2020 Oct;7(Suppl 3):S212-S221. doi: 10.5152/eurjrheum.2020.19124. Epub 2020 Jul 6. Eur J Rheumatol. 2020. PMID: 33164735 Free PMC article. Review.
-
Aesthetic Treatment Considerations in Patients with Cutaneous Autoimmune Disease.J Clin Aesthet Dermatol. 2025 Jul 1;18(7):26-29. J Clin Aesthet Dermatol. 2025. PMID: 40778011 Free PMC article. Review.
-
Locoregional Treatments for Digital Ulcers in Systemic Sclerosis: A Systematic Review.Acta Derm Venereol. 2021 Jun 22;101(6):adv00478. doi: 10.2340/00015555-3839. Acta Derm Venereol. 2021. PMID: 34043013 Free PMC article.
-
Toxin for Treating Raynaud Conditions in Hands (The TORCH Study): A Systematic Review and Meta-analysis.Plast Reconstr Surg Glob Open. 2024 Jun 14;12(6):e5885. doi: 10.1097/GOX.0000000000005885. eCollection 2024 Jun. Plast Reconstr Surg Glob Open. 2024. PMID: 38881966 Free PMC article.
References
-
- Cappelli L, Wigley FM. Management of Raynaud Phenomenon and Digital Ulcers in Scleroderma. Rheumatic diseases clinics of North America. 2015;41(3):419–38. - PubMed
-
- Block JA, Sequeira W. Raynaud's phenomenon. Lancet (London, England) 2001;357(9273):2042–8. - PubMed
-
- Wigley FM, Flavahan NA. Raynaud's Phenomenon. New England Journal of Medicine. 2016;375(6):556–65. - PubMed
-
- Frantz C, Avouac J, Distler O, Amrouche F, Godard D, Kennedy AT, et al. Impaired quality of life in systemic sclerosis and patient perception of the disease: A large international survey. Seminars in arthritis and rheumatism. 2016 - PubMed
-
- Hummers LK, Wigley FM. Management of Raynaud's phenomenon and digital ischemic lesions in scleroderma. Rheumatic diseases clinics of North America. 2003;29(2):293–313. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

