Pretreatment serum uracil concentration as a predictor of severe and fatal fluoropyrimidine-associated toxicity
- PMID: 28427087
- PMCID: PMC5520099
- DOI: 10.1038/bjc.2017.94
Pretreatment serum uracil concentration as a predictor of severe and fatal fluoropyrimidine-associated toxicity
Abstract
Background: We investigated the predictive value of dihydropyrimidine dehydrogenase (DPD) phenotype, measured as pretreatment serum uracil and dihydrouracil concentrations, for severe as well as fatal fluoropyrimidine-associated toxicity in 550 patients treated previously with fluoropyrimidines during a prospective multicenter study.
Methods: Pretreatment serum concentrations of uracil and dihydrouracil were measured using a validated LC-MS/MS method. The primary endpoint of this analysis was global (any) severe fluoropyrimidine-associated toxicity, that is, grade ⩾3 toxicity according to the NCI CTC-AE v3.0, occurring during the first cycle of treatment. The predictive value of uracil and the uracil/dihydrouracil ratio for early severe fluoropyrimidine-associated toxicity were compared. Pharmacogenetic variants in DPYD (c.2846A>T, c.1679T>G, c.1129-5923C>G, and c.1601G>A) and TYMS (TYMS 5'-UTR VNTR and TYMS 3'-UTR 6-bp ins/del) were measured and tested for associations with severe fluoropyrimidine-associated toxicity to compare predictive value with DPD phenotype. The Benjamini-Hochberg false discovery rate method was used to control for type I errors at level q<0.050 (corresponding to P<0.010).
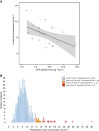
Results: Uracil was superior to the dihydrouracil/uracil ratio as a predictor of severe toxicity. High pretreatment uracil concentrations (>16 ng ml-1) were strongly associated with global severe toxicity (OR 5.3, P=0.009), severe gastrointestinal toxicity (OR 33.7, P<0.0001), toxicity-related hospitalisation (OR 16.9, P<0.0001), as well as fatal treatment-related toxicity (OR 44.8, P=0.001). None of the DPYD variants alone, or TYMS variants alone, were associated with severe toxicity.
Conclusions: High pretreatment uracil concentration was strongly predictive of severe, including fatal, fluoropyrimidine-associated toxicity, and is a highly promising phenotypic marker to identify patients at risk of severe fluoropyrimidine-associated toxicity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Amstutz U, Offer SM, Sistonen J, Joerger M, Diasio RB, Largiadèr CR (2015) Polymorphisms in MIR27A Associated with Early-Onset Toxicity in Fluoropyrimidine-Based Chemotherapy. Clin Cancer Res 21: 2038–2044. - PubMed
-
- Benjamini Y, Hochberg Y (1995) Controlling the False Discovery Rate: a Practical and Powerful Approach to Multiple Testing. J R Stat Soc B 57: 289–300.
-
- Boisdron-Celle M, Remaud G, Traore S, Poirier AL, Gamelin L, Morel A, Gamelin E (2007) 5-Fluorouracil-related severe toxicity: a comparison of different methods for the pretherapeutic detection of dihydropyrimidine dehydrogenase deficiency. Cancer Lett 249: 271–282. - PubMed
-
- Ciccolini J, Mercier C, Blachon M-F, Favre R, Durand A, Lacarelle B (2004) A simple and rapid high-performance liquid chromatographic (HPLC) method for 5-fluorouracil (5-FU) assay in plasma and possible detection of patients with impaired dihydropyrimidine dehydrogenase (DPD) activity. J Clin Pharm Ther 29: 307–315. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

