The amelioration of cartilage degeneration by photo-crosslinked GelHA hydrogel and crizotinib encapsulated chitosan microspheres
- PMID: 28427172
- PMCID: PMC5444739
- DOI: 10.18632/oncotarget.15750
The amelioration of cartilage degeneration by photo-crosslinked GelHA hydrogel and crizotinib encapsulated chitosan microspheres
Abstract
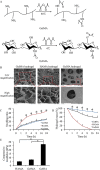
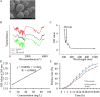
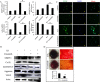
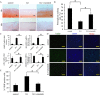
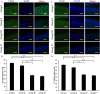
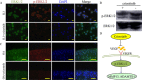
The present study aimed to investigate the synergistic therapeutic effect of decreaseing cartilage angiogenesis via exposure to crizotinib encapsulated by chitosan microspheres and photo-crosslinked hydrogel, with the goal of evaluating crizotinib as a treatment for osteoarthritis. First, we developed and evaluated the characteristics of hydrogels and chitosan microspheres. Next, we measured the effect of crizotinib on the cartilage degeneration induced by interleukin-1β in chondrocytes. Crizotinib ameliorated the pathological changes induced by interleukin-1β via its anti-angiogenesis function. In addition, we surgically induced osteoarthritis in mice, which were then injected intra-articularly with crizotinib-loaded biomaterials. Cartilage matrix degradation, expression of vascular endothelial growth factor and extracellular signal-regulated kinases 1/2 were evaluated after surgery. Treatment with the combination of crizotinib-loaded biomaterials retarded the progression of surgically induced osteoarthritis. Crizotinib ameliorated cartilage matrix degradation by promoting anti-angiogenesis and impeding extracellular signal-regulated kinases 1/2 signaling pathway. Our results demonstrate that the combination of photo-crosslinked hydrogel and crizotinib-loaded chitosan microspheres might represent a promising strategy for osteoarthritis treatment.
Keywords: angiogenesis; hydrogel; osteoarthritis.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures



Similar articles
-
Photo-crosslinked HAMA hydrogel with cordycepin encapsulated chitosan microspheres for osteoarthritis treatment.Oncotarget. 2017 Jan 10;8(2):2835-2849. doi: 10.18632/oncotarget.13748. Oncotarget. 2017. PMID: 27926509 Free PMC article.
-
Intra-articular delivery of sinomenium encapsulated by chitosan microspheres and photo-crosslinked GelMA hydrogel ameliorates osteoarthritis by effectively regulating autophagy.Biomaterials. 2016 Mar;81:1-13. doi: 10.1016/j.biomaterials.2015.12.006. Epub 2015 Dec 15. Biomaterials. 2016. PMID: 26713680
-
Enhanced osteoarthritis treatment using an injectable pH-responsive and cartilage-targeted liposome-anchored kartogenin-incorporated methacrylated gelatin hydrogel microspheres.Int J Biol Macromol. 2025 Jun;313:144198. doi: 10.1016/j.ijbiomac.2025.144198. Epub 2025 May 15. Int J Biol Macromol. 2025. PMID: 40379185
-
Indian Hedgehog, a critical modulator in osteoarthritis, could be a potential therapeutic target for attenuating cartilage degeneration disease.Connect Tissue Res. 2014 Aug;55(4):257-61. doi: 10.3109/03008207.2014.925885. Epub 2014 Jun 13. Connect Tissue Res. 2014. PMID: 24844414 Review.
-
Markers of osteoarthritis and cartilage research in animal models.Curr Opin Rheumatol. 1993 Jul;5(4):494-502. doi: 10.1097/00002281-199305040-00015. Curr Opin Rheumatol. 1993. PMID: 8251023 Review.
Cited by
-
Potential and recent advances of microcarriers in repairing cartilage defects.J Orthop Translat. 2021 Jan 13;27:101-109. doi: 10.1016/j.jot.2020.10.005. eCollection 2021 Mar. J Orthop Translat. 2021. PMID: 33520655 Free PMC article. Review.
-
The Role of Gene Therapy in Cartilage Repair.Arch Bone Jt Surg. 2019 Mar;7(2):79-90. Arch Bone Jt Surg. 2019. PMID: 31211186 Free PMC article. Review.
References
-
- Pap T, Korb-Pap A. Cartilage damage in osteoarthritis and rheumatoid arthritis—two unequal siblings. Nat Rev Rheumatol. 2015;11:606–15. - PubMed
-
- Dahaghin S, Tehrani-Banihashemi SA, Faezi ST, Jamshidi AR, Davatchi F. Squatting, sitting on the floor, or cycling: are life-long daily activities risk factors for clinical knee osteoarthritis? Stage III results of a community-based study. Arthritis Rheum. 2009;61:1337–42. - PubMed
-
- Gallagher B, Tjoumakaris FP, Harwood MI, Good RP, Ciccotti MG, Freedman KB. Chondroprotection and the prevention of osteoarthritis progression of the knee: a systematic review of treatment agents. Am J Sports Med. 2015;43:734–44. - PubMed
-
- Kikuchi R, Nakamura K, MacLauchlan S, Ngo DT, Shimizu I, Fuster JJ, Katanasaka Y, Yoshida S, Qiu Y, Yamaguchi TP, Matsushita T, Murohara T, Gokce N, et al. An antiangiogenic isoform of VEGF-A contributes to impaired vascularization in peripheral artery disease. Nat Med. 2014;20:1464–71. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

