Assessment of the interlaboratory variability and robustness of JAK2V617F mutation assays: A study involving a consortium of 19 Italian laboratories
- PMID: 28427233
- PMCID: PMC5464813
- DOI: 10.18632/oncotarget.15940
Assessment of the interlaboratory variability and robustness of JAK2V617F mutation assays: A study involving a consortium of 19 Italian laboratories
Abstract
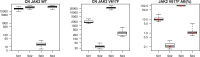
To date, a plenty of techniques for the detection of JAK2V617F is used over different laboratories, with substantial differences in specificity and sensitivity. Therefore, to provide reliable and comparable results, the standardization of molecular techniques is mandatory.A network of 19 centers was established to 1) evaluate the inter- and intra-laboratory variability in JAK2V617F quantification, 2) identify the most robust assay for the standardization of the molecular test and 3) allow consistent interpretation of individual patient analysis results. The study was conceived in 3 different rounds, in which all centers had to blindly test DNA samples with different JAK2V617F allele burden (AB) using both quantitative and qualitative assays.The positivity of samples with an AB < 1% was not detected by qualitative assays. Conversely, laboratories performing the quantitative approach were able to determine the expected JAK2V617F AB. Quantitative results were reliable across all mutation loads with moderate variability at low AB (0.1 and 1%; CV = 0.46 and 0.77, respectively). Remarkably, all laboratories clearly distinguished between the 0.1 and 1% mutated samples.In conclusion, a qualitative approach is not sensitive enough to detect the JAK2V617F mutation, especially at low AB. On the contrary, the ipsogen JAK2 MutaQuant CE-IVD kit resulted in a high, efficient and sensitive quantification detection of all mutation loads. This study sets the basis for the standardization of molecular techniques for JAK2V617F determination, which will require the employment of approved operating procedures and the use of certificated standards, such as the recent WHO 1st International Reference Panel for Genomic JAK2V617F.
Keywords: JAK2 V617F mutation; molecular diagnosis; myeloproliferative neoplasms; qPCR standardization.
Conflict of interest statement
L. Orlandi, F. Cassavia and F. Orsini are employed by Werfen; V. Laloux is employed by QIAGEN.
None of the co-authors, participating laboratories or institutions received any payments for being involved in this study.
Figures


References
-
- Tefferi A, Vardiman JW. Classification and diagnosis of myeloproliferative neoplasms: the 2008 World Health Organization criteria and point-of-care diagnostic algorithms. Leukemia. 2008;22:14–22. - PubMed
-
- Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, Bloomfield CD, Cazzola M, Vardiman JW. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391–405. - PubMed
-
- Klampfl T, Gisslinger H, Harutyunyan AS, Nivarthi H, Rumi E, Milosevic JD, Them NC, Berg T, Gisslinger B, Pietra D, Chen D, Vladimer GI, Bagienski K, et al. Somatic mutations of calreticulin in myeloproliferative neoplasms. N Engl J Med. 2013;369:2379–90. - PubMed
-
- Bench AJ, White HE, Foroni L, Godfrey AL, Gerrard G, Akiki S, Awan A, Carter I, Goday-Fernandez A, Langabeer SE, Clench T, Clark J, Evans PA, et al. British Committee for Standards in Haematology. Molecular diagnosis of the myeloproliferative neoplasms: UK guidelines for the detection of JAK2 V617F and other relevant mutations. Br J Haematol. 2013;160:25–34. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

