Oxidative DNA double strand breaks and autophagy in the antitumor effect of sterically hindered platinum(II) complexes in NSCLCs
- PMID: 28427237
- PMCID: PMC5458179
- DOI: 10.18632/oncotarget.15944
Oxidative DNA double strand breaks and autophagy in the antitumor effect of sterically hindered platinum(II) complexes in NSCLCs
Abstract
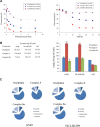
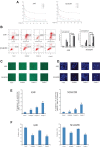
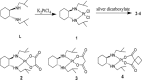
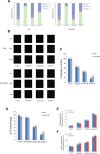
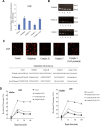
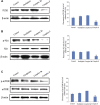
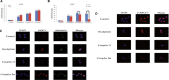
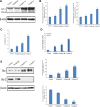
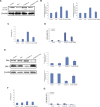
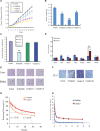
A series of novel platinum(II) complexes with (1R,2R)-N1,N2-diisobutyl-1,2-diaminocyclohexane as a carrier ligand, while N1,N2-diisobutyl moiety serving as steric hindrance were designed, synthesized and characterized. The in vitro biological assays demonstrated that complex 3 had increased cytotoxicity against lung cancer cells, especially non-small-cell lung cancer (NSCLC) compared to its mono-substituted complex 3a, indicating that the sterically hindered alkyl moieties have significant influences on its antitumor property. However, the mechanism still remains unclear. The further studies revealed that complex 3 could induce ROS overproduction, severe DNA double strands breaks and inhibit the activation of DNA damage repair proteins within nucleus, leading to cell-cycle arrest and cell death. Moreover, complex 3 could induce autophagy via the accumulation of autophagic vacuoles and alterations of autophagic protein expression. Interestingly, the ROS scavengers, N-acetyl-cysteine (NAC) could reverse complex 3-induced DNA double strands breaks and autophagic responses more significantly compared to complex 3a. The results demonstrated that the ROS generation plays an important role in the DNA double strands breaks and autophagic responses in the antitumor effect of complex 3 with N1,N2-diisobutyl moiety. Our study offered a novel therapeutic strategy and put new insights into the anticancer research of the complexes with N1,N2-diisobutyl moiety served as steric hindrance.
Keywords: MDC1/aprataxin; N1, N2-diisobutyl moiety; ROS; double strand breaks; platinum(II) complexes.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Luteoloside induces G0/G1 arrest and pro-death autophagy through the ROS-mediated AKT/mTOR/p70S6K signalling pathway in human non-small cell lung cancer cell lines.Biochem Biophys Res Commun. 2017 Dec 9;494(1-2):263-269. doi: 10.1016/j.bbrc.2017.10.042. Epub 2017 Oct 9. Biochem Biophys Res Commun. 2017. PMID: 29024631
-
Cytotoxicity profile of novel sterically hindered platinum(II) complexes with (1R,2R)-N(1),N(2)-dibutyl-1,2-diaminocyclohexane.Eur J Med Chem. 2015;96:187-95. doi: 10.1016/j.ejmech.2015.04.019. Epub 2015 Apr 8. Eur J Med Chem. 2015. PMID: 25874342
-
Palladium(ii) and platinum(ii) saccharinate complexes with bis(diphenylphosphino)methane/ethane: synthesis, S-phase arrest and ROS-mediated apoptosis in human colon cancer cells.Dalton Trans. 2018 Aug 21;47(33):11397-11410. doi: 10.1039/c8dt02389a. Dalton Trans. 2018. PMID: 30062356
-
Induction of reactive oxygen species: an emerging approach for cancer therapy.Apoptosis. 2017 Nov;22(11):1321-1335. doi: 10.1007/s10495-017-1424-9. Apoptosis. 2017. PMID: 28936716 Review.
-
Antitumor potential of platinum(II) complexes of selenium donor ligands.Metallomics. 2023 Apr 3;15(4):mfad020. doi: 10.1093/mtomcs/mfad020. Metallomics. 2023. PMID: 36918206 Review.
Cited by
-
Preclinical Therapeutic Assessment of a New Chemotherapeutics [Dichloro(4,4'-Bis(2,2,3,3-Tetrafluoropropoxy) Methyl)-2,2'-Bipryridine) Platinum] in an Orthotopic Patient-Derived Xenograft Model of Triple-Negative Breast Cancers.Pharmaceutics. 2022 Apr 11;14(4):839. doi: 10.3390/pharmaceutics14040839. Pharmaceutics. 2022. PMID: 35456673 Free PMC article.
-
Unlocking the dual role of autophagy: A new strategy for treating lung cancer.J Pharm Anal. 2025 Mar;15(3):101098. doi: 10.1016/j.jpha.2024.101098. Epub 2024 Sep 12. J Pharm Anal. 2025. PMID: 40104173 Free PMC article. Review.
References
-
- Rosenberg B, VanCamp L, Trosko JE, Mansour VH. Platinum compounds: a new class of potent anti-tumor agents. Nature. 1969;222:385–386. - PubMed
-
- Kelland L. The resurgence of platinum-based cancer chemotherapy. Nat Rev Cancer. 2007;7:573–584. - PubMed
-
- Wang XY, Wang XH, Guo ZJ. Functionalization of platinum complexes for biomedical applications. Acc Chem Res. 2015;48:2622–2631. - PubMed
-
- Liu FF, Gou SH, Chen FH, Fang L, Zhao J. Study on antitumor platinum(II) complexes of chiral diamines with dicyclic species as steric hindrance. J Med Chem. 2015;58:6368–6377. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

