Premenstrual Dysphoric Disorder Symptoms Following Ovarian Suppression: Triggered by Change in Ovarian Steroid Levels But Not Continuous Stable Levels
- PMID: 28427285
- PMCID: PMC5624833
- DOI: 10.1176/appi.ajp.2017.16101113
Premenstrual Dysphoric Disorder Symptoms Following Ovarian Suppression: Triggered by Change in Ovarian Steroid Levels But Not Continuous Stable Levels
Abstract
Objective: Premenstrual dysphoric disorder (PMDD) symptoms are eliminated by ovarian suppression and stimulated by administration of ovarian steroids, yet they appear with ovarian steroid levels indistinguishable from those in women without PMDD. Thus, symptoms could be precipitated either by an acute change in ovarian steroid levels or by stable levels above a critical threshold playing a permissive role in expression of an underlying infradian affective "pacemaker." The authors attempted to determine which condition triggers PMDD symptoms.
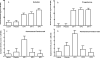
Method: The study included 22 women with PMDD, ages 30 to 50 years. Twelve women who experienced symptom remission after 2-3 months of GnRH agonist-induced ovarian suppression (leuprolide) then received 1 month of single-blind (participant only) placebo and then 3 months of continuous combined estradiol/progesterone. Primary outcome measures were the Rating for Premenstrual Tension observer and self-ratings completed every 2 weeks during clinic visits. Multivariate repeated-measure ANOVA for mixed models was employed.
Results: Both self- and observer-rated scores on the Rating for Premenstrual Tension were significantly increased (more symptomatic) during the first month of combined estradiol/progesterone compared with the last month of leuprolide alone, the placebo month, and the second and third months of estradiol/progesterone. There were no significant differences in symptom severity between the last month of leuprolide alone, placebo month, or second and third months of estradiol/progesterone. Finally, the Rating for Premenstrual Tension scores in the second and third estradiol/progesterone months did not significantly differ.
Conclusions: The findings demonstrate that the change in estradiol/progesterone levels from low to high, and not the steady-state level, was associated with onset of PMDD symptoms. Therapeutic efforts to modulate the change in steroid levels proximate to ovulation merit further study.
Trial registration: ClinicalTrials.gov NCT00005011.
Keywords: Premenstrual Syndrome; estradiol; progesterone.
Conflict of interest statement
The Authors have no potential conflicts of interest or financial support regarding this manuscript.
Figures

Comment in
-
Stable hormones for stable moods.Sci Transl Med. 2017 May 24;9(391):eaan4293. doi: 10.1126/scitranslmed.aan4293. Sci Transl Med. 2017. PMID: 28539473
-
For Women With PMDD, Acute Changes in Ovarian Steroids, But Not Enduring Higher Levels, Precipitate Symptoms.Am J Psychiatry. 2017 Oct 1;174(10):917-919. doi: 10.1176/appi.ajp.2017.17070803. Am J Psychiatry. 2017. PMID: 28965460 No abstract available.
References
-
- American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders. Fourth. Washington, DC: American Psychiatric Association; 1994.
-
- American Psychiatric Association Fifth Edition . American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders. Fifth. Arlington, VA: 2013.
-
- Rubinow DR, Schmidt PJ. Gonadal steroid regulation of mood: the lessons of premenstrual syndrome. Frontiers in Neuroendocrinology. 2006;27:210–216. - PubMed
-
- Brown CS, Ling FW, Andersen RN, Farmer RG, Arheart KL. Efficacy of depot leuprolide in premenstrual syndrome: effect of symptom severity and type in a controlled trial. Obstetrics and Gynecology. 1994;84:779–786. - PubMed
-
- Helvacioglu A, Yeoman RR, Hazelton JM, Aksel S. Premenstrual syndrome and related hormonal changes: long-acting gonadotropin releasing hormone agonist treatment. Journal of Reproductive Medicine. 1993;38:864–870. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

