Lack of interleukin-13 receptor α1 delays the loss of dopaminergic neurons during chronic stress
- PMID: 28427412
- PMCID: PMC5399344
- DOI: 10.1186/s12974-017-0862-1
Lack of interleukin-13 receptor α1 delays the loss of dopaminergic neurons during chronic stress
Abstract
Background: The majority of Parkinson's disease (PD) cases are sporadic and idiopathic suggesting that this neurodegenerative disorder is the result of both environmental and genetic factors. Stress and neuroinflammation are among the factors being investigated for their possible contributions to PD. Experiments in rodents showed that severe chronic stress can reduce the number of dopaminergic neurons in the substantia nigra pars compacta (SNc); the same cells that are lost in PD. These actions are at least in part mediated by increased oxidative stress. Here, we tested the hypothesis that the interleukin-13 receptor alpha 1 (IL-13Rα1), a cytokine receptor whose activation increases the vulnerability of dopaminergic neurons to oxidative damage, participates in the stress-dependent damage of these neurons.
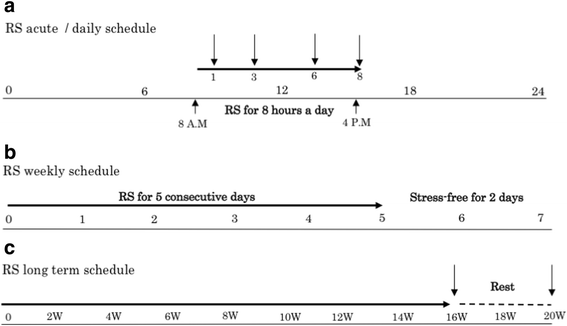
Methods: Mice were subject to daily sessions of 8 h (acute) stress for 16 weeks (5 days a week), a procedure previously showed to induce loss of dopaminergic neurons in the SNc. The source and the kinetics of interleukin-13 (IL-13), the endogenous ligand of IL-13Rα1, were evaluated 0, 1, 3, 6, and 8 h and at 16 weeks of stress. Identification of IL-13 producing cell-type was performed by immunofluorescent and by in situ hybridization experiments. Markers of oxidative stress, microglia activation, and the number of dopaminergic neurons in IL-13Rα1 knock-out animals (Il13ra1 Y/ - ) and their wild-type littermates (Il13ra1 Y/+ ) were evaluated at 16 weeks of stress and at 20 weeks, following a 4 week non-stressed period and compared to non-stressed mice.
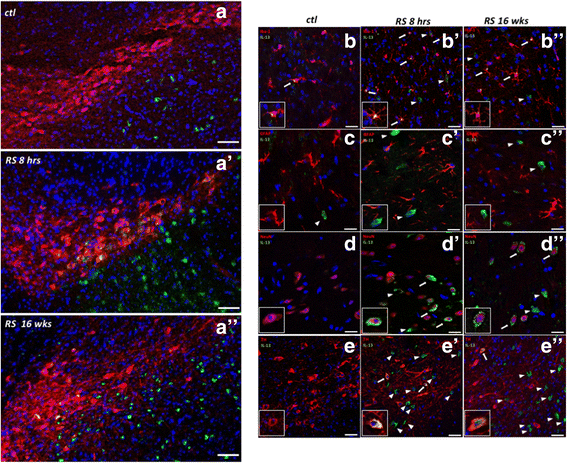
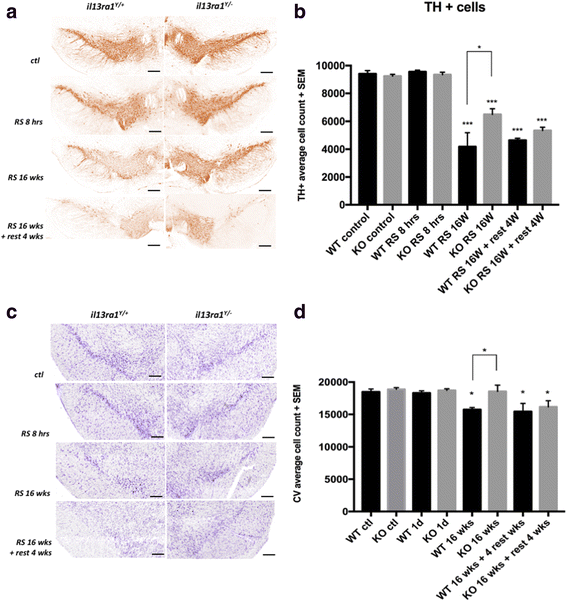
Results: IL-13 was expressed in microglial cells within the SN and in a fraction of the tyrosine hydroxylase-positive neurons in the SNc. IL-13 levels were elevated during daily stress and peaked at 6 h. 16 weeks of chronic restraint stress significantly reduced the number of SNc dopaminergic neurons in Il13ra1 Y/+ mice. Neuronal loss at 16 weeks was significantly lower in Il13ra1 Y/- mice. However, the loss of dopaminergic neurons measured at 20 weeks, after 4 weeks of non-stress following the 16 weeks of stress, was similar in Il13ra1 Y/+ and Il13ra1 Y/- mice.
Conclusions: IL-13, a cytokine previously demonstrated to increase the susceptibility of SNc dopaminergic neurons to oxidative stress, is elevated in the SN by restraint stress. Lack of IL-13Rα1 did not prevent nor halted but delayed neuronal loss in the mouse model of chronic restraint stress. IL-13/IL-13Rα1 may represent a target to reduce the rate of DA neuronal loss that can occur during severe chronic restraint stress.
Keywords: Interleukin; Microglia; Neuroinflammation; Oxidative stress; Parkinson’s disease; Stress.
Figures


Similar articles
-
Cutting edge: IL-13Rα1 expression in dopaminergic neurons contributes to their oxidative stress-mediated loss following chronic peripheral treatment with lipopolysaccharide.J Immunol. 2012 Dec 15;189(12):5498-502. doi: 10.4049/jimmunol.1102150. Epub 2012 Nov 19. J Immunol. 2012. PMID: 23169588 Free PMC article.
-
Loss of collapsin response mediator protein 4 suppresses dopaminergic neuron death in an 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced mouse model of Parkinson's disease.J Neurochem. 2016 Jun;137(5):795-805. doi: 10.1111/jnc.13617. Epub 2016 Apr 12. J Neurochem. 2016. PMID: 26991935
-
Loss of dopaminergic neurons occurs in the ventral tegmental area and hypothalamus of rats following chronic stress: Possible pathogenetic loci for depression involved in Parkinson's disease.Neurosci Res. 2016 Oct;111:48-55. doi: 10.1016/j.neures.2016.04.008. Epub 2016 Apr 30. Neurosci Res. 2016. PMID: 27142317
-
Converging roles of ion channels, calcium, metabolic stress, and activity pattern of Substantia nigra dopaminergic neurons in health and Parkinson's disease.J Neurochem. 2016 Oct;139 Suppl 1(Suppl Suppl 1):156-178. doi: 10.1111/jnc.13572. Epub 2016 Mar 23. J Neurochem. 2016. PMID: 26865375 Free PMC article. Review.
-
Parkinson's Disease: Cells Succumbing to Lifelong Dopamine-Related Oxidative Stress and Other Bioenergetic Challenges.Int J Mol Sci. 2024 Feb 7;25(4):2009. doi: 10.3390/ijms25042009. Int J Mol Sci. 2024. PMID: 38396687 Free PMC article. Review.
Cited by
-
Interleukin 13 signaling modulates dopaminergic functions and nicotine reward in rodents.Mol Psychiatry. 2025 Aug 7. doi: 10.1038/s41380-025-03137-3. Online ahead of print. Mol Psychiatry. 2025. PMID: 40775068
-
Differentiation and regulation of CD4+ T cell subsets in Parkinson's disease.Cell Mol Life Sci. 2024 Aug 17;81(1):352. doi: 10.1007/s00018-024-05402-0. Cell Mol Life Sci. 2024. PMID: 39153043 Free PMC article. Review.
-
Interrelationship and Sequencing of Interleukins4, 13, 31, and 33 - An Integrated Systematic Review: Dermatological and Multidisciplinary Perspectives.J Inflamm Res. 2022 Sep 8;15:5163-5184. doi: 10.2147/JIR.S374060. eCollection 2022. J Inflamm Res. 2022. PMID: 36110506 Free PMC article. Review.
-
Neuroprotective effect of L-DOPA-induced interleukin-13 on striatonigral degeneration in cerebral ischemia.Cell Death Dis. 2024 Nov 22;15(11):854. doi: 10.1038/s41419-024-07252-x. Cell Death Dis. 2024. PMID: 39578419 Free PMC article.
-
Profiling the neuroimmune cascade in 3xTg-AD mice exposed to successive mild traumatic brain injuries.J Neuroinflammation. 2024 Jun 13;21(1):156. doi: 10.1186/s12974-024-03128-1. J Neuroinflammation. 2024. PMID: 38872143 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

