Divergent Function of Programmed Death-Ligand 1 in Donor Tissue versus Recipient Immune System in a Murine Model of Bronchiolitis Obliterans
- PMID: 28427861
- PMCID: PMC5455059
- DOI: 10.1016/j.ajpath.2017.02.007
Divergent Function of Programmed Death-Ligand 1 in Donor Tissue versus Recipient Immune System in a Murine Model of Bronchiolitis Obliterans
Abstract
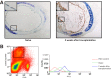
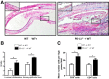
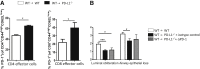
Costimulatory molecules, such as the programmed death ligand (PD-L1), might exert differential effects on T-cell function, depending on the clinical setting and/or immunological environment. Given the impact of T cells on bronchiolitis obliterans (BO) in lung transplantation, we used an established tracheal transplant model inducing BO-like lesions to investigate the impact of PD-L1 on alloimmune responses and histopathological outcome in BO. In contrast to other transplant models in which PD-L1 generally shows protective functions, we demonstrated that PD-L1 has divergent effects depending on its location in donor versus recipient tissue. Although PD-L1 deficiency in donor tissue worsened histopathological outcome, and increased systemic inflammatory response, recipient PD-L1 deficiency induced opposite effects. Mechanistic studies revealed PD-L1-deficient recipients were hyporesponsive toward alloantigen, despite increased numbers of CD8+ effector T cells. The function of PD-L1 on T cells after unspecific stimulation was dependent on both cell type and strength of stimulation. This novel function of recipient PD-L1 may result from the high degree of T-cell activation within the highly immunogenic milieu of the transplanted tissue. In this model, both decreased T-cell alloimmune responses and the reduction of BO in PD-L1-deficient recipients suggest a potential therapeutic role of selectively blocking PD-L1 in the recipient. Further investigation is warranted to determine the impact of this finding embedded in the complex pathophysiological context of BO.
Copyright © 2017 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures






Similar articles
-
The function of donor versus recipient programmed death-ligand 1 in corneal allograft survival.J Immunol. 2007 Sep 15;179(6):3672-9. doi: 10.4049/jimmunol.179.6.3672. J Immunol. 2007. PMID: 17785803
-
Analysis of the role of negative T cell costimulatory pathways in CD4 and CD8 T cell-mediated alloimmune responses in vivo.J Immunol. 2005 Jun 1;174(11):6648-56. doi: 10.4049/jimmunol.174.11.6648. J Immunol. 2005. PMID: 15905503
-
Adenosine signaling via the adenosine 2B receptor is involved in bronchiolitis obliterans development.J Heart Lung Transplant. 2010 Dec;29(12):1405-14. doi: 10.1016/j.healun.2010.07.005. J Heart Lung Transplant. 2010. PMID: 20920842 Free PMC article.
-
Cytotoxicity of Natural Killer Cells Activated Through NKG2D Contributes to the Development of Bronchiolitis Obliterans in a Murine Heterotopic Tracheal Transplant Model.Am J Transplant. 2017 Sep;17(9):2338-2349. doi: 10.1111/ajt.14257. Epub 2017 Apr 21. Am J Transplant. 2017. PMID: 28251796
-
The role of adenosine A2A receptor signaling in bronchiolitis obliterans.Ann Thorac Surg. 2009 Oct;88(4):1071-8. doi: 10.1016/j.athoracsur.2009.06.032. Ann Thorac Surg. 2009. PMID: 19766783 Free PMC article.
Cited by
-
In Situ Immune Profiling of Heart Transplant Biopsies Improves Diagnostic Accuracy and Rejection Risk Stratification.JACC Basic Transl Sci. 2020 Apr 1;5(4):328-340. doi: 10.1016/j.jacbts.2020.01.015. eCollection 2020 Apr. JACC Basic Transl Sci. 2020. PMID: 32368693 Free PMC article.
-
PLK1 Inhibition alleviates transplant-associated obliterative bronchiolitis by suppressing myofibroblast differentiation.Aging (Albany NY). 2020 Jun 15;12(12):11636-11652. doi: 10.18632/aging.103330. Epub 2020 Jun 15. Aging (Albany NY). 2020. PMID: 32541091 Free PMC article.
References
-
- Li X.C., Rothstein D.M., Sayegh M.H. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. Immunol Rev. 2009;229:271–293. - PubMed
-
- Alegre M.L., Najafian N. Costimulatory molecules as targets for the induction of transplantation tolerance. Curr Mol Med. 2006;6:843–857. - PubMed
-
- Greenwald R.J., Freeman G.J., Sharpe A.H. The B7 family revisited. Annu Rev Immunol. 2005;23:515–548. - PubMed
-
- Chen L. Co-inhibitory molecules of the B7-CD28 family in the control of T-cell immunity. Nat Rev Immunol. 2004;4:336–347. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

