ROS in Cancer: The Burning Question
- PMID: 28427863
- PMCID: PMC5462452
- DOI: 10.1016/j.molmed.2017.03.004
ROS in Cancer: The Burning Question
Abstract
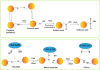
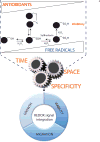
An unanswered question in human health is whether antioxidation prevents or promotes cancer. Antioxidation has historically been viewed as chemopreventive, but emerging evidence suggests that antioxidants may be supportive of neoplasia. We posit this contention to be rooted in the fact that ROS do not operate as one single biochemical entity, but as diverse secondary messengers in cancer cells. This cautions against therapeutic strategies to increase ROS at a global level. To leverage redox alterations towards the development of effective therapies necessitates the application of biophysical and biochemical approaches to define redox dynamics and to functionally elucidate specific oxidative modifications in cancer versus normal cells. An improved understanding of the sophisticated workings of redox biology is imperative to defeating cancer.
Copyright © 2017 Elsevier Ltd. All rights reserved.
Figures

References
-
- The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. The Alpha-Tocopherol, Beta Carotene Cancer Prevention Study Group. N Engl J Med. 1994;330:1029–1035. - PubMed
-
- Adam-Vizi V, Chinopoulos C. Bioenergetics and the formation of mitochondrial reactive oxygen species. Trends in pharmacological sciences. 2006;27:639–645. - PubMed
-
- Albrecht SC, Barata AG, Grosshans J, Teleman AA, Dick TP. In vivo mapping of hydrogen peroxide and oxidized glutathione reveals chemical and regional specificity of redox homeostasis. Cell Metab. 2011;14:819–829. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

