Klotho, an antiaging molecule, attenuates oxidant-induced alveolar epithelial cell mtDNA damage and apoptosis
- PMID: 28428174
- PMCID: PMC5538874
- DOI: 10.1152/ajplung.00063.2017
Klotho, an antiaging molecule, attenuates oxidant-induced alveolar epithelial cell mtDNA damage and apoptosis
Abstract
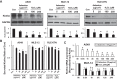
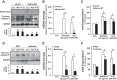
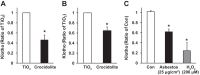
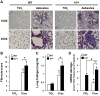
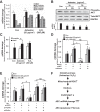
Alveolar epithelial cell (AEC) apoptosis and inadequate repair resulting from "exaggerated" lung aging and mitochondrial dysfunction are critical determinants promoting lung fibrosis. α-Klotho, which is an antiaging molecule that is expressed predominantly in the kidney and secreted in the blood, can protect lung epithelial cells against hyperoxia-induced apoptosis. We reasoned that Klotho protects AEC exposed to oxidative stress in part by maintaining mitochondrial DNA (mtDNA) integrity and mitigating apoptosis. We find that Klotho levels are decreased in both serum and alveolar type II (AT2) cells from asbestos-exposed mice. We show that oxidative stress reduces AEC Klotho mRNA and protein expression, whereas Klotho overexpression is protective while Klotho silencing augments AEC mtDNA damage. Compared with wild-type, Klotho heterozygous hypomorphic allele (kl/+) mice have increased asbestos-induced lung fibrosis due in part to increased AT2 cell mtDNA damage. Notably, we demonstrate that serum Klotho levels are reduced in wild-type but not mitochondrial catalase overexpressing (MCAT) mice 3 wk following exposure to asbestos and that EUK-134, a MnSOD/catalase mimetic, mitigates oxidant-induced reductions in AEC Klotho expression. Using pharmacologic and genetic silencing studies, we show that Klotho attenuates oxidant-induced AEC mtDNA damage and apoptosis via mechanisms dependent on AKT activation arising from upstream fibroblast growth factor receptor 1 activation. Our findings suggest that Klotho preserves AEC mtDNA integrity in the setting of oxidative stress necessary for preventing apoptosis and asbestos-induced lung fibrosis. We reason that strategies aimed at augmenting AEC Klotho levels may be an innovative approach for mitigating age-related lung diseases.
Keywords: Klotho; alveolar epithelial cell; mitochondrial DNA damage; oxidative stress; pulmonary fibrosis.
Figures
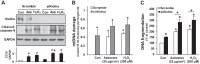

Similar articles
-
Mitochondrial 8-oxoguanine DNA glycosylase mitigates alveolar epithelial cell PINK1 deficiency, mitochondrial DNA damage, apoptosis, and lung fibrosis.Am J Physiol Lung Cell Mol Physiol. 2020 May 1;318(5):L1084-L1096. doi: 10.1152/ajplung.00069.2019. Epub 2020 Mar 25. Am J Physiol Lung Cell Mol Physiol. 2020. PMID: 32209025 Free PMC article.
-
Mitochondrial catalase overexpressed transgenic mice are protected against lung fibrosis in part via preventing alveolar epithelial cell mitochondrial DNA damage.Free Radic Biol Med. 2016 Dec;101:482-490. doi: 10.1016/j.freeradbiomed.2016.11.007. Epub 2016 Nov 11. Free Radic Biol Med. 2016. PMID: 27840320 Free PMC article.
-
SIRT3 deficiency promotes lung fibrosis by augmenting alveolar epithelial cell mitochondrial DNA damage and apoptosis.FASEB J. 2017 Jun;31(6):2520-2532. doi: 10.1096/fj.201601077R. Epub 2017 Mar 3. FASEB J. 2017. PMID: 28258190 Free PMC article.
-
The Role of Mitochondrial DNA in Mediating Alveolar Epithelial Cell Apoptosis and Pulmonary Fibrosis.Int J Mol Sci. 2015 Sep 7;16(9):21486-519. doi: 10.3390/ijms160921486. Int J Mol Sci. 2015. PMID: 26370974 Free PMC article. Review.
-
Molecular mechanisms of asbestos-induced lung epithelial cell apoptosis.Chem Biol Interact. 2010 Nov 5;188(2):309-18. doi: 10.1016/j.cbi.2010.03.047. Epub 2010 Apr 7. Chem Biol Interact. 2010. PMID: 20380827 Review.
Cited by
-
Mitochondrial 8-oxoguanine DNA glycosylase mitigates alveolar epithelial cell PINK1 deficiency, mitochondrial DNA damage, apoptosis, and lung fibrosis.Am J Physiol Lung Cell Mol Physiol. 2020 May 1;318(5):L1084-L1096. doi: 10.1152/ajplung.00069.2019. Epub 2020 Mar 25. Am J Physiol Lung Cell Mol Physiol. 2020. PMID: 32209025 Free PMC article.
-
Advances in cellular senescence in idiopathic pulmonary fibrosis (Review).Exp Ther Med. 2023 Feb 15;25(4):145. doi: 10.3892/etm.2023.11844. eCollection 2023 Apr. Exp Ther Med. 2023. PMID: 36911379 Free PMC article. Review.
-
Role of DNA methylation transferase in urinary system diseases: From basic to clinical perspectives (Review).Int J Mol Med. 2025 Feb;55(2):19. doi: 10.3892/ijmm.2024.5460. Epub 2024 Nov 22. Int J Mol Med. 2025. PMID: 39575487 Free PMC article. Review.
-
Mechanisms of progressive fibrosis in connective tissue disease (CTD)-associated interstitial lung diseases (ILDs).Ann Rheum Dis. 2021 Feb;80(2):143-150. doi: 10.1136/annrheumdis-2020-217230. Epub 2020 Oct 9. Ann Rheum Dis. 2021. PMID: 33037004 Free PMC article. Review.
-
Mechanisms and consequences of oxidative stress in lung disease: therapeutic implications for an aging populace.Am J Physiol Lung Cell Mol Physiol. 2018 Apr 1;314(4):L642-L653. doi: 10.1152/ajplung.00275.2017. Epub 2017 Dec 14. Am J Physiol Lung Cell Mol Physiol. 2018. PMID: 29351446 Free PMC article. Review.
References
-
- Asai O, Nakatani K, Tanaka T, Sakan H, Imura A, Yoshimoto S, Samejima K, Yamaguchi Y, Matsui M, Akai Y, Konishi N, Iwano M, Nabeshima Y, Saito Y. Decreased renal α-Klotho expression in early diabetic nephropathy in humans and mice and its possible role in urinary calcium excretion. Kidney Int 81: 539–547, 2012. doi: 10.1038/ki.2011.423. - DOI - PubMed
-
- Bueno M, Lai YC, Romero Y, Brands J, St Croix CM, Kamga C, Corey C, Herazo-Maya JD, Sembrat J, Lee JS, Duncan SR, Rojas M, Shiva S, Chu CT, Mora AL. PINK1 deficiency impairs mitochondrial homeostasis and promotes lung fibrosis. J Clin Invest 125: 521–538, 2015. doi: 10.1172/JCI74942. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

