PD-L1 Expression in Melanoma: A Quantitative Immunohistochemical Antibody Comparison
- PMID: 28428193
- PMCID: PMC6175606
- DOI: 10.1158/1078-0432.CCR-16-1821
PD-L1 Expression in Melanoma: A Quantitative Immunohistochemical Antibody Comparison
Abstract
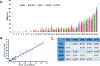
Purpose: PD-L1 expression in the pretreatment tumor microenvironment enriches for response to anti-PD-1/PD-L1 therapies. The purpose of this study was to quantitatively compare the performance of five monoclonal anti-PD-L1 antibodies used in recent landmark publications.Experimental Design: PD-L1 IHC was performed on 34 formalin-fixed paraffin-embedded archival melanoma samples using the 5H1, SP142, 28-8, 22C3, and SP263 clones. The percentage of total cells (including melanocytes and immune cells) demonstrating cell surface PD-L1 staining, as well as intensity measurements/H-scores, were assessed for each melanoma specimen using a computer-assisted platform. Staining properties were compared between antibodies.Results: Strong correlations were observed between the percentage of PD-L1(+) cells across all clones studied (R2 = 0.81-0.96). When present, discordant results were attributable to geographic heterogeneity of the melanoma tissue section rather than differences in PD-L1 antibody staining characteristics. PD-L1 intensity/H-scores strongly correlated with percentage of PD-L1(+) cells (R2 > 0.78, all clones).Conclusions: The 5H1, SP142, 28-8, 22C3, and SP263 clones all demonstrated similar performance characteristics when used in a standardized IHC assay on melanoma specimens. Reported differences in PD-L1 IHC assays using these antibodies are thus most likely due to assay characteristics beyond the antibody itself. Our findings also argue against the inclusion of an intensity/H-score in chromogenic PD-L1 IHC assays. Clin Cancer Res; 23(16); 4938-44. ©2017 AACR.
©2017 American Association for Cancer Research.
Conflict of interest statement
COI for RAA: Consultant (compensated): Adaptive Biotech; Research Funding from Merck and BMS. For EJL: Consultant (compensated): Bristol-Myers Squibb, EMD Serono, Merck, Novartis; Research funding: AstraZeneca, Genetech, Merck. For JMT: Consultant (compensated) Bristol-Myers Squibb, Merck, AstraZeneca; Research Funding from BMS.
Figures


References
-
- Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 2015;372:320–30. - PubMed
-
- Weber JS, D’Angelo SP, Minor D, Hodi FS, Gutzmer R, Neyns B, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti- CTLA-4 treatment (CheckMate 037): a randomized, controlled, open-label, phase 3 trial. Lancet Oncol 2015;16:375–84. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

