Precision physiology and rescue of brain ion channel disorders
- PMID: 28428202
- PMCID: PMC5412535
- DOI: 10.1085/jgp.201711759
Precision physiology and rescue of brain ion channel disorders
Abstract
Ion channel genes, originally implicated in inherited excitability disorders of muscle and heart, have captured a major role in the molecular diagnosis of central nervous system disease. Their arrival is heralded by neurologists confounded by a broad phenotypic spectrum of early-onset epilepsy, autism, and cognitive impairment with few effective treatments. As detection of rare structural variants in channel subunit proteins becomes routine, it is apparent that primary sequence alone cannot reliably predict clinical severity or pinpoint a therapeutic solution. Future gains in the clinical utility of variants as biomarkers integral to clinical decision making and drug discovery depend on our ability to unravel complex developmental relationships bridging single ion channel structure and human physiology.
© 2017 Noebels.
Figures
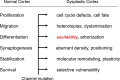
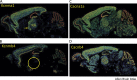


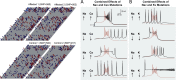
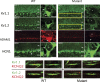

References
-
- Bechi G., Rusconi R., Cestèle S., Striano P., Franceschetti S., and Mantegazza M.. 2015. Rescuable folding defective NaV1.1 (SCN1A) mutants in epilepsy: properties, occurrence, and novel rescuing strategy with peptides targeted to the endoplasmic reticulum. Neurobiol. Dis. 75:100–114. 10.1016/j.nbd.2014.12.028 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

