TSLP-activated dendritic cells induce human T follicular helper cell differentiation through OX40-ligand
- PMID: 28428203
- PMCID: PMC5413322
- DOI: 10.1084/jem.20150402
TSLP-activated dendritic cells induce human T follicular helper cell differentiation through OX40-ligand
Abstract
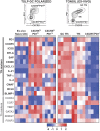
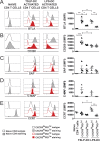
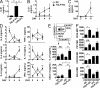
T follicular helper cells (Tfh) are important regulators of humoral responses. Human Tfh polarization pathways have been thus far associated with Th1 and Th17 polarization pathways. How human Tfh cells differentiate in Th2-skewed environments is unknown. We show that thymic stromal lymphopoietin (TSLP)-activated dendritic cells (DCs) promote human Tfh differentiation from naive CD4 T cells. We identified a novel population, distinct from Th2 cells, expressing IL-21 and TNF, suggestive of inflammatory cells. TSLP-induced T cells expressed CXCR5, CXCL13, ICOS, PD1, BCL6, BTLA, and SAP, among other Tfh markers. Functionally, TSLP-DC-polarized T cells induced IgE secretion by memory B cells, and this depended on IL-4Rα. TSLP-activated DCs stimulated circulating memory Tfh cells to produce IL-21 and CXCL13. Mechanistically, TSLP-induced Tfh differentiation depended on OX40-ligand, but not on ICOS-ligand. Our results delineate a pathway of human Tfh differentiation in Th2 environments.
© 2017 Pattarini et al.
Figures







References
-
- Briot A., Deraison C., Lacroix M., Bonnart C., Robin A., Besson C., Dubus P., and Hovnanian A.. 2009. Kallikrein 5 induces atopic dermatitis-like lesions through PAR2-mediated thymic stromal lymphopoietin expression in Netherton syndrome. J. Exp. Med. 206:1135–1147. 10.1084/jem.20082242 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

