Imaging Manifestations of Pseudoprogression in Metastatic Melanoma Nodes Injected with Talimogene Laherparepvec: Initial Experience
- PMID: 28428211
- PMCID: PMC7960096
- DOI: 10.3174/ajnr.A5206
Imaging Manifestations of Pseudoprogression in Metastatic Melanoma Nodes Injected with Talimogene Laherparepvec: Initial Experience
Abstract
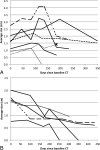
Talimogene laherparepvec is an oncolytic virus recently approved for targeted treatment of advanced melanoma. Because of an inflammatory reaction, treated lesions may increase in size and develop infiltrative margins that can be construed as disease progression or extracapsular spread. In this report, we describe our initial experience imaging the response of metastatic nodes injected with talimogene laherparepvec. Six of 12 nodes (50%) showed growth from baseline followed by decreased size, 5 of 12 nodes (42%) showed a downward size trend, and 1 node showed continued increase in size. Seven of 9 nodes (78%) developed infiltrative margins at a median of 79 days, and 6 of 9 (67%) nodes became necrotic at a median of 76 days after injection, all showing decreased size at final follow-up. An increase in the size of nodes injected with talimogene laherparepvec does not necessarily indicate progression. Infiltrative margins are also frequently seen and may be confused with extracapsular disease.
© 2017 by American Journal of Neuroradiology.
Figures


References
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
