Deficiency of Cholesteryl Ester Transfer Protein Protects Against Atherosclerosis in Rabbits
- PMID: 28428219
- PMCID: PMC5805397
- DOI: 10.1161/ATVBAHA.117.309114
Deficiency of Cholesteryl Ester Transfer Protein Protects Against Atherosclerosis in Rabbits
Abstract
Objective: CETP (cholesteryl ester transfer protein) plays an important role in lipoprotein metabolism; however, whether inhibition of CETP activity can prevent cardiovascular disease remains controversial.
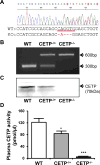
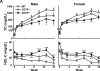
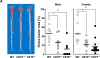
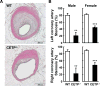
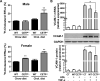
Approach and results: We generated CETP knockout (KO) rabbits by zinc finger nuclease gene editing and compared their susceptibility to cholesterol diet-induced atherosclerosis to that of wild-type (WT) rabbits. On a chow diet, KO rabbits showed higher plasma levels of high-density lipoprotein (HDL) cholesterol than WT controls, and HDL particles of KO rabbits were essentially rich in apolipoprotein AI and apolipoprotein E contents. When challenged with a cholesterol-rich diet for 18 weeks, KO rabbits not only had higher HDL cholesterol levels but also lower total cholesterol levels than WT rabbits. Analysis of plasma lipoproteins revealed that reduced plasma total cholesterol in KO rabbits was attributable to decreased apolipoprotein B-containing particles, while HDLs remained higher than that in WT rabbits. Both aortic and coronary atherosclerosis was significantly reduced in KO rabbits compared with WT rabbits. Apolipoprotein B-depleted plasma isolated from CETP KO rabbits showed significantly higher capacity for cholesterol efflux from macrophages than that from WT rabbits. Furthermore, HDLs isolated from CETP KO rabbits suppressed tumor necrosis factor-α-induced vascular cell adhesion molecule 1 and E-selectin expression in cultured endothelial cells.
Conclusions: These results provide evidence that genetic ablation of CETP activity protects against cholesterol diet-induced atherosclerosis in rabbits.
Keywords: apolipoprotein; atherosclerosis; cholesterol reduction; cholesteryl ester transfer protein genetics; high-density lipoprotein.
© 2017 American Heart Association, Inc.
Figures
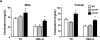
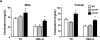



Similar articles
-
Apolipoprotein CIII Deficiency Protects Against Atherosclerosis in Knockout Rabbits.Arterioscler Thromb Vasc Biol. 2020 Sep;40(9):2095-2107. doi: 10.1161/ATVBAHA.120.314368. Epub 2020 Aug 6. Arterioscler Thromb Vasc Biol. 2020. PMID: 32757647 Free PMC article.
-
Involvement of CETP (Cholesteryl Ester Transfer Protein) in the Shift of Sphingosine-1-Phosphate Among Lipoproteins and in the Modulation of its Functions.Arterioscler Thromb Vasc Biol. 2017 Mar;37(3):506-514. doi: 10.1161/ATVBAHA.116.308692. Epub 2017 Jan 26. Arterioscler Thromb Vasc Biol. 2017. PMID: 28126827
-
ADCY9 (Adenylate Cyclase Type 9) Inactivation Protects From Atherosclerosis Only in the Absence of CETP (Cholesteryl Ester Transfer Protein).Circulation. 2018 Oct 16;138(16):1677-1692. doi: 10.1161/CIRCULATIONAHA.117.031134. Circulation. 2018. PMID: 29674325
-
Re-evaluation of cholesteryl ester transfer protein function in atherosclerosis based upon genetics and pharmacological manipulation.Curr Opin Lipidol. 2016 Oct;27(5):459-72. doi: 10.1097/MOL.0000000000000332. Curr Opin Lipidol. 2016. PMID: 27454452 Review.
-
Cholesteryl ester transfer protein: a novel target for raising HDL and inhibiting atherosclerosis.Arterioscler Thromb Vasc Biol. 2003 Feb 1;23(2):160-7. doi: 10.1161/01.atv.0000054658.91146.64. Arterioscler Thromb Vasc Biol. 2003. PMID: 12588754 Review.
Cited by
-
Non-Mouse Models of Atherosclerosis: Approaches to Exploring the Translational Potential of New Therapies.Int J Mol Sci. 2022 Oct 26;23(21):12964. doi: 10.3390/ijms232112964. Int J Mol Sci. 2022. PMID: 36361754 Free PMC article. Review.
-
Rabbit models of human diseases for diagnostics and therapeutics development.Dev Comp Immunol. 2019 Mar;92:99-104. doi: 10.1016/j.dci.2018.10.003. Epub 2018 Oct 16. Dev Comp Immunol. 2019. PMID: 30339876 Free PMC article. Review.
-
Integration of Transformative Platforms for the Discovery of Causative Genes in Cardiovascular Diseases.Cardiovasc Drugs Ther. 2021 Jun;35(3):637-654. doi: 10.1007/s10557-021-07175-1. Epub 2021 Apr 15. Cardiovasc Drugs Ther. 2021. PMID: 33856594 Free PMC article. Review.
-
Regulation of cholesterol homeostasis in health and diseases: from mechanisms to targeted therapeutics.Signal Transduct Target Ther. 2022 Aug 2;7(1):265. doi: 10.1038/s41392-022-01125-5. Signal Transduct Target Ther. 2022. PMID: 35918332 Free PMC article. Review.
-
Endothelial Lipase Exerts its Anti-Atherogenic Effect through Increased Catabolism of β-VLDLs.J Atheroscler Thromb. 2021 Feb 1;28(2):157-168. doi: 10.5551/jat.55244. Epub 2020 May 23. J Atheroscler Thromb. 2021. PMID: 32448826 Free PMC article.
References
-
- Gordon T, Castelli WP, Hjortland MC, Kannel WB, Dawber TR. High-density lipoprotein as a protective factor against coronary heart-disease - framingham study. Am J Med. 1977;62:707–714. - PubMed
-
- Tall AR. Plasma cholesteryl ester transfer protein. Journal of lipid research. 1993;34:1255–1274. - PubMed
-
- Barter PJ, Nicholls SJ, Kastelein JJ, Rye KA. Is cholesteryl ester transfer protein inhibition an effective strategy to reduce cardiovascular risk? Cetp inhibition as a strategy to reduce cardiovascular risk: The pro case. Circulation. 2015;132:423–432. - PubMed
-
- Brown ML, Inazu A, Hesler CB, Agellon LB, Mann C, Whitlock ME, Marcel YL, Milne RW, Koizumi J, Mabuchi H, Takeda R, Tall AR. Molecular basis of lipid transfer protein deficiency in a family with increased high-density lipoproteins. Nature. 1989;342:448–451. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

