Glutamatergic Mechanisms Involved in Bladder Overactivity and Pudendal Neuromodulation in Cats
- PMID: 28428223
- PMCID: PMC5454588
- DOI: 10.1124/jpet.117.240895
Glutamatergic Mechanisms Involved in Bladder Overactivity and Pudendal Neuromodulation in Cats
Abstract
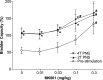
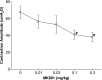
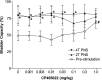
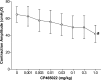
The involvement of ionotropic glutamate receptors in bladder overactivity and pudendal neuromodulation was determined in α-chloralose anesthetized cats by intravenously administering MK801 (a NMDA receptor antagonist) or CP465022 (an AMPA receptor antagonist). Infusion of 0.5% acetic acid (AA) into the bladder produced bladder overactivity. In the first group of 5 cats, bladder capacity was significantly (P < 0.05) reduced to 55.3±10.0% of saline control by AA irritation. Pudendal nerve stimulation (PNS) significantly (P < 0.05) increased bladder capacity to 106.8 ± 15.0% and 106.7 ± 13.3% of saline control at 2T and 4T intensity, respectively. T is threshold intensity for inducing anal twitching. MK801 at 0.3 mg/kg prevented the increase in capacity by 2T or 4T PNS. In the second group of 5 cats, bladder capacity was significantly (P < 0.05) reduced to 49.0 ± 7.5% of saline control by AA irritation. It was then significantly (P < 0.05) increased to 80.8±13.5% and 79.0±14.0% of saline control by 2T and 4T PNS, respectively. CP465022 at 0.03-1 mg/kg prevented the increase in capacity by 2T PNS and at 0.3-1 mg/kg prevented the increase in capacity by 4T PNS. In both groups, MK801 at 0.3 mg/kg and CP465022 at 1 mg/kg significantly (P < 0.05) increased the prestimulation bladder capacity (about 80% and 20%, respectively) and reduced the amplitude of bladder contractions (about 30 and 20 cmH2O, respectively). These results indicate that NMDA and AMPA glutamate receptors are important for PNS to inhibit bladder overactivity and that tonic activation of these receptors also contributes to the bladder overactivity induced by AA irritation.
Copyright © 2017 by The American Society for Pharmacology and Experimental Therapeutics.
Figures


Similar articles
-
Role of spinal GABAA receptors in pudendal inhibition of nociceptive and nonnociceptive bladder reflexes in cats.Am J Physiol Renal Physiol. 2014 Apr 1;306(7):F781-9. doi: 10.1152/ajprenal.00679.2013. Epub 2014 Feb 12. Am J Physiol Renal Physiol. 2014. PMID: 24523385 Free PMC article.
-
Role of cannabinoid receptor type 1 in tibial and pudendal neuromodulation of bladder overactivity in cats.Am J Physiol Renal Physiol. 2017 Mar 1;312(3):F482-F488. doi: 10.1152/ajprenal.00586.2016. Epub 2016 Dec 7. Am J Physiol Renal Physiol. 2017. PMID: 27927655 Free PMC article.
-
Effects of duloxetine and WAY100635 on pudendal inhibition of bladder overactivity in cats.J Pharmacol Exp Ther. 2014 Jun;349(3):402-7. doi: 10.1124/jpet.113.211557. Epub 2014 Mar 25. J Pharmacol Exp Ther. 2014. PMID: 24667547 Free PMC article.
-
Role of spinal metabotropic glutamate receptor 5 in pudendal inhibition of the nociceptive bladder reflex in cats.Am J Physiol Renal Physiol. 2015 Apr 15;308(8):F832-8. doi: 10.1152/ajprenal.00623.2014. Epub 2015 Feb 11. Am J Physiol Renal Physiol. 2015. PMID: 25673810 Free PMC article.
-
Sex difference in the contribution of GABAB receptors to tibial neuromodulation of bladder overactivity in cats.Am J Physiol Regul Integr Comp Physiol. 2017 Mar 1;312(3):R292-R300. doi: 10.1152/ajpregu.00401.2016. Epub 2016 Dec 14. Am J Physiol Regul Integr Comp Physiol. 2017. PMID: 27974317 Free PMC article.
Cited by
-
Spinal interneuronal mechanisms underlying pudendal and tibial neuromodulation of bladder function in cats.Exp Neurol. 2018 Oct;308:100-110. doi: 10.1016/j.expneurol.2018.06.015. Epub 2018 Jul 7. Exp Neurol. 2018. PMID: 30017972 Free PMC article.
-
Disruption of circadian rhythm as a potential pathogenesis of nocturia.Nat Rev Urol. 2025 May;22(5):276-293. doi: 10.1038/s41585-024-00961-0. Epub 2024 Nov 14. Nat Rev Urol. 2025. PMID: 39543359 Free PMC article. Review.
-
Suppression of Urinary Voiding by Conditional High Frequency Stimulation of the Pelvic Nerve in Conscious Rats.Front Physiol. 2018 Apr 30;9:437. doi: 10.3389/fphys.2018.00437. eCollection 2018. Front Physiol. 2018. PMID: 29760663 Free PMC article.
-
CoQ10 ameliorates monosodium glutamate-induced alteration in detrusor activity and responsiveness in rats via anti-inflammatory, anti-oxidant and channel inhibiting mechanisms.BMC Urol. 2019 Oct 28;19(1):103. doi: 10.1186/s12894-019-0534-9. BMC Urol. 2019. PMID: 31660941 Free PMC article.
-
The mechanism of action of neuromodulation in the treatment of overactive bladder.Nat Rev Urol. 2025 Jul;22(7):414-426. doi: 10.1038/s41585-024-00967-8. Epub 2024 Dec 9. Nat Rev Urol. 2025. PMID: 39653756 Review.
References
-
- Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U, van Kerrebroeck P, Victor A, Wein A. (2002) The standardisation of terminology of lower urinary tract function: report from the Standardisation Sub-committee of the International Continence Society. Am J Obstet Gynecol 187:116–126. - PubMed
-
- Andersson KE, Wein AJ. (2004) Pharmacology of the lower urinary tract: basis for current and future treatments of urinary incontinence. Pharmacol Rev 56:581–631. - PubMed
-
- Araki I, De Groat WC. (1996) Unitary excitatory synaptic currents in preganglionic neurons mediated by two distinct groups of interneurons in neonatal rat sacral parasympathetic nucleus. J Neurophysiol 76:215–226. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

