A novel physiological role for ARF1 in the formation of bidirectional tubules from the Golgi
- PMID: 28428254
- PMCID: PMC5469610
- DOI: 10.1091/mbc.E16-12-0863
A novel physiological role for ARF1 in the formation of bidirectional tubules from the Golgi
Abstract
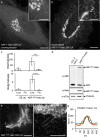
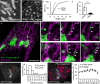
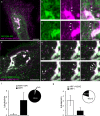
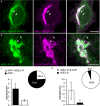
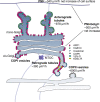
Capitalizing on CRISPR/Cas9 gene-editing techniques and super-resolution nanoscopy, we explore the role of the small GTPase ARF1 in mediating transport steps at the Golgi. Besides its well-established role in generating COPI vesicles, we find that ARF1 is also involved in the formation of long (∼3 µm), thin (∼110 nm diameter) tubular carriers. The anterograde and retrograde tubular carriers are both largely free of the classical Golgi coat proteins coatomer (COPI) and clathrin. Instead, they contain ARF1 along their entire length at a density estimated to be in the range of close packing. Experiments using a mutant form of ARF1 affecting GTP hydrolysis suggest that ARF1[GTP] is functionally required for the tubules to form. Dynamic confocal and stimulated emission depletion imaging shows that ARF1-rich tubular compartments fall into two distinct classes containing 1) anterograde cargoes and clathrin clusters or 2) retrograde cargoes and coatomer clusters.
© 2017 Bottanelli et al. This article is distributed by The American Society for Cell Biology under license from the author(s). Two months after publication it is available to the public under an Attribution–Noncommercial–Share Alike 3.0 Unported Creative Commons License (http://creativecommons.org/licenses/by-nc-sa/3.0).
Figures



Similar articles
-
Multiple and stepwise interactions between coatomer and ADP-ribosylation factor-1 (Arf1)-GTP.Traffic. 2007 May;8(5):582-93. doi: 10.1111/j.1600-0854.2007.00554.x. Traffic. 2007. PMID: 17451557
-
Dissection of COPI and Arf1 dynamics in vivo and role in Golgi membrane transport.Nature. 2002 May 9;417(6885):187-93. doi: 10.1038/417187a. Nature. 2002. PMID: 12000962
-
COPI vesicles accumulating in the presence of a GTP restricted arf1 mutant are depleted of anterograde and retrograde cargo.J Cell Sci. 2000 Jan;113 ( Pt 1):135-44. doi: 10.1242/jcs.113.1.135. J Cell Sci. 2000. PMID: 10591632
-
Membrane curvature and the control of GTP hydrolysis in Arf1 during COPI vesicle formation.Biochem Soc Trans. 2005 Aug;33(Pt 4):619-22. doi: 10.1042/BST0330619. Biochem Soc Trans. 2005. PMID: 16042557 Review.
-
ARF1 and SAR1 GTPases in endomembrane trafficking in plants.Int J Mol Sci. 2013 Sep 5;14(9):18181-99. doi: 10.3390/ijms140918181. Int J Mol Sci. 2013. PMID: 24013371 Free PMC article. Review.
Cited by
-
Acylation - A New Means to Control Traffic Through the Golgi.Front Cell Dev Biol. 2019 Jun 12;7:109. doi: 10.3389/fcell.2019.00109. eCollection 2019. Front Cell Dev Biol. 2019. PMID: 31245373 Free PMC article. Review.
-
Characterization of lipoprotein lipase storage vesicles in 3T3-L1 adipocytes.J Cell Sci. 2022 Mar 1;135(5):jcs258734. doi: 10.1242/jcs.258734. Epub 2021 Aug 12. J Cell Sci. 2022. PMID: 34382637 Free PMC article.
-
STED imaging of endogenously tagged ARF GTPases reveals their distinct nanoscale localizations.J Cell Biol. 2023 Jul 3;222(7):e202205107. doi: 10.1083/jcb.202205107. Epub 2023 Apr 27. J Cell Biol. 2023. PMID: 37102998 Free PMC article.
-
Arf GTPase-activating proteins SMAP1 and AGFG2 regulate the size of Weibel-Palade bodies and exocytosis of von Willebrand factor.Biol Open. 2021 Sep 15;10(9):bio058789. doi: 10.1242/bio.058789. Epub 2021 Sep 1. Biol Open. 2021. PMID: 34369554 Free PMC article.
-
Spatial control of AMPK signaling at subcellular compartments.Crit Rev Biochem Mol Biol. 2020 Feb;55(1):17-32. doi: 10.1080/10409238.2020.1727840. Epub 2020 Feb 18. Crit Rev Biochem Mol Biol. 2020. PMID: 32069425 Free PMC article. Review.
References
-
- Abramoff MD, Magalhaes PJ, Ram SJ. Image processing with ImageJ. Biophotonics Int. 2004;11:36–42.
-
- Barlowe C, Helenius A. Cargo capture and bulk flow in the early secretory pathway. Annu Rev Cell Dev Biol. 2016;32:197–222. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

