Deletion of inositol-requiring enzyme-1α in podocytes disrupts glomerular capillary integrity and autophagy
- PMID: 28428258
- PMCID: PMC5469607
- DOI: 10.1091/mbc.E16-12-0828
Deletion of inositol-requiring enzyme-1α in podocytes disrupts glomerular capillary integrity and autophagy
Abstract
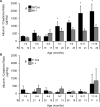
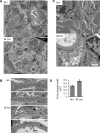
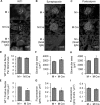
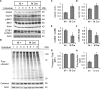
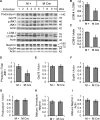
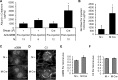
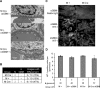
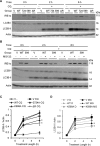
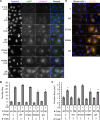
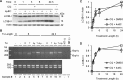
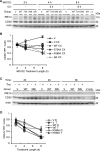
Inositol-requiring enzyme-1α (IRE1α) is an endoplasmic reticulum (ER)-transmembrane endoribonuclease kinase that plays an essential function in extraembryonic tissues during normal development and is activated during ER stress. To address the functional role of IRE1α in glomerular podocytes, we produced podocyte-specific IRE1α-deletion mice. In male mice, deletion of IRE1α in podocytes resulted in albuminuria beginning at 5 mo of age and worsening with time. Electron microscopy revealed focal podocyte foot-process effacement in 9-mo-old male IRE1α-deletion mice, as well as microvillous transformation of podocyte plasma membranes. Compared with control, glomerular cross-sectional and capillary lumenal areas were greater in deletion mice, and there was relative podocyte depletion. Levels of microtubule-associated protein 1A/1B-light chain 3 (LC3)-II expression and c-Jun N-terminal kinase-1 phosphorylation were decreased in IRE1α-deletion glomeruli, in keeping with reduced autophagy. Deletion of IRE1α exacerbated glomerular injury in anti-glomerular basement membrane nephritis. In cell culture, IRE1α dominant-negative mutants reduced the physiological (basal) accumulation of LC3B-II and the size of autophagic vacuoles but did not affect ER-associated degradation. Thus IRE1α is essential for maintaining podocyte and glomerular integrity as mice age and in glomerulonephritis. The mechanism is related, at least in part, to the maintenance of autophagy in podocytes.
© 2017 Kaufman et al. This article is distributed by The American Society for Cell Biology under license from the author(s). Two months after publication it is available to the public under an Attribution–Noncommercial–Share Alike 3.0 Unported Creative Commons License (http://creativecommons.org/licenses/by-nc-sa/3.0).
Figures

Similar articles
-
Ste20-like kinase, SLK, a novel mediator of podocyte integrity.Am J Physiol Renal Physiol. 2018 Jul 1;315(1):F186-F198. doi: 10.1152/ajprenal.00238.2017. Epub 2017 Nov 29. Am J Physiol Renal Physiol. 2018. PMID: 29187370 Free PMC article.
-
Deletion of IRE1α in podocytes exacerbates diabetic nephropathy in mice.Sci Rep. 2024 May 22;14(1):11718. doi: 10.1038/s41598-024-62599-7. Sci Rep. 2024. PMID: 38778209 Free PMC article.
-
The unfolded protein response transducer IRE1α promotes reticulophagy in podocytes.Biochim Biophys Acta Mol Basis Dis. 2022 Jun 1;1868(6):166391. doi: 10.1016/j.bbadis.2022.166391. Epub 2022 Mar 15. Biochim Biophys Acta Mol Basis Dis. 2022. PMID: 35304860
-
Beyond UPR: cell-specific roles of ER stress sensor IRE1α in kidney ischemic injury and transplant rejection.Kidney Int. 2023 Sep;104(3):463-469. doi: 10.1016/j.kint.2023.06.016. Epub 2023 Jun 28. Kidney Int. 2023. PMID: 37391039 Free PMC article. Review.
-
IRE1α Implications in Endoplasmic Reticulum Stress-Mediated Development and Pathogenesis of Autoimmune Diseases.Front Immunol. 2018 Jun 6;9:1289. doi: 10.3389/fimmu.2018.01289. eCollection 2018. Front Immunol. 2018. PMID: 29928282 Free PMC article. Review.
Cited by
-
Recent Advances in Proteinuric Kidney Disease/Nephrotic Syndrome: Lessons from Knockout/Transgenic Mouse Models.Biomedicines. 2023 Jun 23;11(7):1803. doi: 10.3390/biomedicines11071803. Biomedicines. 2023. PMID: 37509442 Free PMC article. Review.
-
Endoplasmic Reticulum Stress in Systemic Lupus Erythematosus and Lupus Nephritis: Potential Therapeutic Target.J Immunol Res. 2023 Aug 31;2023:7625817. doi: 10.1155/2023/7625817. eCollection 2023. J Immunol Res. 2023. PMID: 37692838 Free PMC article. Review.
-
Acute Kidney Injury and Progression of Diabetic Kidney Disease.Adv Chronic Kidney Dis. 2018 Mar;25(2):166-180. doi: 10.1053/j.ackd.2017.12.005. Adv Chronic Kidney Dis. 2018. PMID: 29580581 Free PMC article. Review.
-
Guidelines for the use and interpretation of assays for monitoring autophagy (4th edition)1.Autophagy. 2021 Jan;17(1):1-382. doi: 10.1080/15548627.2020.1797280. Epub 2021 Feb 8. Autophagy. 2021. PMID: 33634751 Free PMC article.
-
Ste20-like kinase, SLK, a novel mediator of podocyte integrity.Am J Physiol Renal Physiol. 2018 Jul 1;315(1):F186-F198. doi: 10.1152/ajprenal.00238.2017. Epub 2017 Nov 29. Am J Physiol Renal Physiol. 2018. PMID: 29187370 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

