Beclin-1-mediated Autophagy Protects Against Cadmium-activated Apoptosis via the Fas/FasL Pathway in Primary Rat Proximal Tubular Cell Culture
- PMID: 28428545
- PMCID: PMC5430518
- DOI: 10.1038/s41598-017-00997-w
Beclin-1-mediated Autophagy Protects Against Cadmium-activated Apoptosis via the Fas/FasL Pathway in Primary Rat Proximal Tubular Cell Culture
Abstract
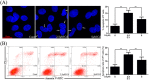
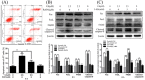
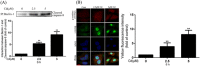
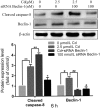
The Fas/FasL signaling pathway is one of the primary apoptosis pathways, but the involvement and regulatory mechanism of this pathway by autophagy remain unclear. Here we demonstrated that cadmium (Cd) activated the Fas/FasL apoptosis pathway in rat proximal tubular (rPT) cells; this was accompanied by simultaneous activation of autophagy resulted in reduced apoptosis. In this model, we induced autophagy through RAPA and further demonstrated that autophagy protects against activation of Fas/FasL signaling and apoptosis. The antiapoptotic effect of autophagy was blocked by 3-MA, an autophagy inhibitor. The interactions between Beclin-1 and Fas, FasL, FADD, caspase-8 and BID/tBID were relatively weak, with the exception of cleaved caspase-8, indicated that minimal interactions between these proteins and Beclin-1 are involved in maintaining the balance of autophagy and apoptosis. Beclin-1 precipitated with cleaved caspase-8 in a dose-dependent mannter, and the expression was increased by siRNA against Beclin-1. These data suggested that Beclin-1-mediated autophagy impairs the expression and function of cleaved caspase-8 to protect against Cd-induced activation of apopotosis through Fas/FasL signaling pathway.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
Cadmium-induced apoptosis in neuronal cells is mediated by Fas/FasL-mediated mitochondrial apoptotic signaling pathway.Sci Rep. 2018 Jun 11;8(1):8837. doi: 10.1038/s41598-018-27106-9. Sci Rep. 2018. PMID: 29891925 Free PMC article.
-
Fluorofenidone inhibits Ang II-induced apoptosis of renal tubular cells through blockage of the Fas/FasL pathway.Int Immunopharmacol. 2011 Sep;11(9):1327-32. doi: 10.1016/j.intimp.2011.04.016. Epub 2011 May 14. Int Immunopharmacol. 2011. PMID: 21586345
-
Trehalose protects against cadmium-induced cytotoxicity in primary rat proximal tubular cells via inhibiting apoptosis and restoring autophagic flux.Cell Death Dis. 2017 Oct 12;8(10):e3099. doi: 10.1038/cddis.2017.475. Cell Death Dis. 2017. PMID: 29022917 Free PMC article.
-
The role of the Fas/FasL signaling pathway in environmental toxicant-induced testicular cell apoptosis: An update.Syst Biol Reprod Med. 2018 Apr;64(2):93-102. doi: 10.1080/19396368.2017.1422046. Epub 2018 Jan 4. Syst Biol Reprod Med. 2018. PMID: 29299971 Review.
-
The Fas/FasL Signaling Pathway: Its Role in the Metastatic Process and as a Target for Treating Osteosarcoma Lung Metastases.Adv Exp Med Biol. 2020;1258:177-187. doi: 10.1007/978-3-030-43085-6_12. Adv Exp Med Biol. 2020. PMID: 32767242 Review.
Cited by
-
BECLIN-1-Mediated Autophagy Suppresses Silica Nanoparticle-Induced Testicular Toxicity via the Inhibition of Caspase 8-Mediated Cell Apoptosis in Leydig Cells.Cells. 2022 Jun 7;11(12):1863. doi: 10.3390/cells11121863. Cells. 2022. PMID: 35740992 Free PMC article.
-
The role of autophagy in metal-induced urogenital carcinogenesis.Semin Cancer Biol. 2021 Nov;76:247-257. doi: 10.1016/j.semcancer.2021.03.022. Epub 2021 Mar 30. Semin Cancer Biol. 2021. PMID: 33798723 Free PMC article. Review.
-
Puerarin Restores Autophagosome-Lysosome Fusion to Alleviate Cadmium-Induced Autophagy Blockade via Restoring the Expression of Rab7 in Hepatocytes.Front Pharmacol. 2021 Apr 14;12:632825. doi: 10.3389/fphar.2021.632825. eCollection 2021. Front Pharmacol. 2021. PMID: 33935722 Free PMC article.
-
A Highly Sensitive "on-off" Time-Resolved Phosphorescence Sensor Based on Aptamer Functionalized Magnetite Nanoparticles for Cadmium Detection in Food Samples.Foods. 2020 Nov 27;9(12):1758. doi: 10.3390/foods9121758. Foods. 2020. PMID: 33261175 Free PMC article.
-
Neuroprotection of photoreceptors by combined inhibition of both Fas and autophagy pathways in P23H mice.Cell Death Dis. 2025 Jul 1;16(1):469. doi: 10.1038/s41419-025-07793-9. Cell Death Dis. 2025. PMID: 40592891 Free PMC article.
References
-
- Luo HF, et al. Analyzing the role of soil and rice cadmium pollution on human renal dysfunction by correlation and path analysis. Environ Sci Pollut Res Int. 2016 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

