The tarantula toxin β/δ-TRTX-Pre1a highlights the importance of the S1-S2 voltage-sensor region for sodium channel subtype selectivity
- PMID: 28428547
- PMCID: PMC5430537
- DOI: 10.1038/s41598-017-01129-0
The tarantula toxin β/δ-TRTX-Pre1a highlights the importance of the S1-S2 voltage-sensor region for sodium channel subtype selectivity
Abstract
Voltage-gated sodium (NaV) channels are essential for the transmission of pain signals in humans making them prime targets for the development of new analgesics. Spider venoms are a rich source of peptide modulators useful to study ion channel structure and function. Here we describe β/δ-TRTX-Pre1a, a 35-residue tarantula peptide that selectively interacts with neuronal NaV channels inhibiting peak current of hNaV1.1, rNaV1.2, hNaV1.6, and hNaV1.7 while concurrently inhibiting fast inactivation of hNaV1.1 and rNaV1.3. The DII and DIV S3-S4 loops of NaV channel voltage sensors are important for the interaction of Pre1a with NaV channels but cannot account for its unique subtype selectivity. Through analysis of the binding regions we ascertained that the variability of the S1-S2 loops between NaV channels contributes substantially to the selectivity profile observed for Pre1a, particularly with regards to fast inactivation. A serine residue on the DIV S2 helix was found to be sufficient to explain Pre1a's potent and selective inhibitory effect on the fast inactivation process of NaV1.1 and 1.3. This work highlights that interactions with both S1-S2 and S3-S4 of NaV channels may be necessary for functional modulation, and that targeting the diverse S1-S2 region within voltage-sensing domains provides an avenue to develop subtype selective tools.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

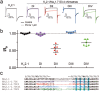

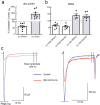
Similar articles
-
Mapping the interaction site for the tarantula toxin hainantoxin-IV (β-TRTX-Hn2a) in the voltage sensor module of domain II of voltage-gated sodium channels.Peptides. 2015 Jun;68:148-56. doi: 10.1016/j.peptides.2014.09.005. Epub 2014 Sep 10. Peptides. 2015. PMID: 25218973
-
Molecular determinant for the tarantula toxin Jingzhaotoxin-I slowing the fast inactivation of voltage-gated sodium channels.Toxicon. 2016 Mar 1;111:13-21. doi: 10.1016/j.toxicon.2015.12.009. Epub 2015 Dec 23. Toxicon. 2016. PMID: 26721415
-
Identification and Characterization of ProTx-III [μ-TRTX-Tp1a], a New Voltage-Gated Sodium Channel Inhibitor from Venom of the Tarantula Thrixopelma pruriens.Mol Pharmacol. 2015 Aug;88(2):291-303. doi: 10.1124/mol.115.098178. Epub 2015 May 15. Mol Pharmacol. 2015. PMID: 25979003
-
A complicated complex: Ion channels, voltage sensing, cell membranes and peptide inhibitors.Neurosci Lett. 2018 Jul 13;679:35-47. doi: 10.1016/j.neulet.2018.04.030. Epub 2018 Apr 21. Neurosci Lett. 2018. PMID: 29684532 Review.
-
Spider and scorpion knottins targeting voltage-gated sodium ion channels in pain signaling.Biochem Pharmacol. 2024 Sep;227:116465. doi: 10.1016/j.bcp.2024.116465. Epub 2024 Aug 3. Biochem Pharmacol. 2024. PMID: 39102991 Review.
Cited by
-
Chemical Synthesis of TFF3 Reveals Novel Mechanistic Insights and a Gut-Stable Metabolite.J Med Chem. 2021 Jul 8;64(13):9484-9495. doi: 10.1021/acs.jmedchem.1c00767. Epub 2021 Jun 18. J Med Chem. 2021. PMID: 34142550 Free PMC article.
-
Structural Conformation and Activity of Spider-Derived Inhibitory Cystine Knot Peptide Pn3a Are Modulated by pH.ACS Omega. 2023 Jul 11;8(29):26276-26286. doi: 10.1021/acsomega.3c02664. eCollection 2023 Jul 25. ACS Omega. 2023. PMID: 37521635 Free PMC article.
-
Spider Knottin Pharmacology at Voltage-Gated Sodium Channels and Their Potential to Modulate Pain Pathways.Toxins (Basel). 2019 Oct 29;11(11):626. doi: 10.3390/toxins11110626. Toxins (Basel). 2019. PMID: 31671792 Free PMC article. Review.
-
The Diversity of Venom: The Importance of Behavior and Venom System Morphology in Understanding Its Ecology and Evolution.Toxins (Basel). 2019 Nov 14;11(11):666. doi: 10.3390/toxins11110666. Toxins (Basel). 2019. PMID: 31739590 Free PMC article. Review.
-
Venom-Derived Peptides Inhibiting Voltage-Gated Sodium and Calcium Channels in Mammalian Sensory Neurons.Adv Exp Med Biol. 2021;1349:3-19. doi: 10.1007/978-981-16-4254-8_1. Adv Exp Med Biol. 2021. PMID: 35138607
References
-
- Catterall, W. A., Goldin, A. L. & Waxman, S. G. Voltage-gated sodium channels, introduction, http://www.guidetopharmacology.org/GRAC/FamilyIntroductionForward?family.... (2016).
-
- Vetter, I. et al. NaV1.7 as a pain target - from gene to pharmacology. Pharmacol. Ther. In press, doi:10.1016/j.pharmthera.2016.11.015 (2016). - PubMed
-
- Wingerd, J. S., Vetter, I. & Lewis, R. J. In Therapeutic Targets 63–122 (John Wiley & Sons, Inc., 2012).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

