Combination of High-density Microelectrode Array and Patch Clamp Recordings to Enable Studies of Multisynaptic Integration
- PMID: 28428560
- PMCID: PMC5430511
- DOI: 10.1038/s41598-017-00981-4
Combination of High-density Microelectrode Array and Patch Clamp Recordings to Enable Studies of Multisynaptic Integration
Abstract
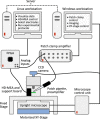
We present a novel, all-electric approach to record and to precisely control the activity of tens of individual presynaptic neurons. The method allows for parallel mapping of the efficacy of multiple synapses and of the resulting dynamics of postsynaptic neurons in a cortical culture. For the measurements, we combine an extracellular high-density microelectrode array, featuring 11'000 electrodes for extracellular recording and stimulation, with intracellular patch-clamp recording. We are able to identify the contributions of individual presynaptic neurons - including inhibitory and excitatory synaptic inputs - to postsynaptic potentials, which enables us to study dendritic integration. Since the electrical stimuli can be controlled at microsecond resolution, our method enables to evoke action potentials at tens of presynaptic cells in precisely orchestrated sequences of high reliability and minimum jitter. We demonstrate the potential of this method by evoking short- and long-term synaptic plasticity through manipulation of multiple synaptic inputs to a specific neuron.
Conflict of interest statement
J.M. and U.F. are co-founders of MaxWell Biosystems AG (Mattenstrasse 26, Basel 4058, Switzerland), commercializing HD-MEAs.
Figures







Similar articles
-
Modeling the Short-Term Dynamics of in Vivo Excitatory Spike Transmission.J Neurosci. 2020 May 20;40(21):4185-4202. doi: 10.1523/JNEUROSCI.1482-19.2020. Epub 2020 Apr 17. J Neurosci. 2020. PMID: 32303648 Free PMC article.
-
Inhibitory synaptic plasticity regulates pyramidal neuron spiking in the rodent hippocampus.Neuroscience. 2008 Jul 31;155(1):64-75. doi: 10.1016/j.neuroscience.2008.05.009. Epub 2008 May 21. Neuroscience. 2008. PMID: 18562122
-
Long-term population spike-timing-dependent plasticity promotes synaptic tagging but not cross-tagging in rat hippocampal area CA1.Proc Natl Acad Sci U S A. 2019 Mar 19;116(12):5737-5746. doi: 10.1073/pnas.1817643116. Epub 2019 Feb 28. Proc Natl Acad Sci U S A. 2019. PMID: 30819889 Free PMC article.
-
Synaptic integration in dendritic trees.J Neurobiol. 2005 Jul;64(1):75-90. doi: 10.1002/neu.20144. J Neurobiol. 2005. PMID: 15884003 Review.
-
Functional dissection of synaptic circuits: in vivo patch-clamp recording in neuroscience.Front Neural Circuits. 2015 May 22;9:23. doi: 10.3389/fncir.2015.00023. eCollection 2015. Front Neural Circuits. 2015. PMID: 26052270 Free PMC article. Review.
Cited by
-
Building Biomimetic Potency Tests for Islet Transplantation.Diabetes. 2021 Feb;70(2):347-363. doi: 10.2337/db20-0297. Diabetes. 2021. PMID: 33472944 Free PMC article. Review.
-
Functional imaging of brain organoids using high-density microelectrode arrays.MRS Bull. 2022;47(6):530-544. doi: 10.1557/s43577-022-00282-w. Epub 2022 Jun 30. MRS Bull. 2022. PMID: 36120104 Free PMC article.
-
Extracellular detection of neuronal coupling.Sci Rep. 2021 Jul 19;11(1):14733. doi: 10.1038/s41598-021-94282-6. Sci Rep. 2021. PMID: 34282275 Free PMC article.
-
A procedure to increase the power of Granger-causal analysis through temporal smoothing.J Neurosci Methods. 2018 Oct 1;308:48-61. doi: 10.1016/j.jneumeth.2018.07.010. Epub 2018 Jul 19. J Neurosci Methods. 2018. PMID: 30031776 Free PMC article.
-
Multisite Attenuated Intracellular Recordings by Extracellular Multielectrode Arrays, a Perspective.Front Neurosci. 2018 Apr 10;12:212. doi: 10.3389/fnins.2018.00212. eCollection 2018. Front Neurosci. 2018. PMID: 29692701 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

