Glucocorticoids Suppress Antimicrobial Autophagy and Nitric Oxide Production and Facilitate Mycobacterial Survival in Macrophages
- PMID: 28428627
- PMCID: PMC5430514
- DOI: 10.1038/s41598-017-01174-9
Glucocorticoids Suppress Antimicrobial Autophagy and Nitric Oxide Production and Facilitate Mycobacterial Survival in Macrophages
Abstract
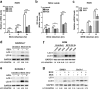
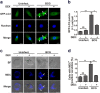
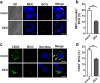
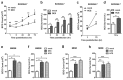
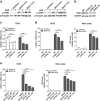
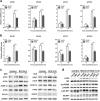
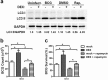
Chronic administration of glucocorticoids has been shown to render individuals highly susceptible to mycobacterial infection and lead to reactivation of latent bacilli. However, the effect of glucocorticoids on innate anti-mycobacterial defense, especially in macrophages remains largely unknown. Here, we found that glucocorticoids inhibited the innate immune response, antimicrobial nitric oxide production and autophagy in mycobacteria-challenged macrophages. Meanwhile, maturation and acidification of mycobacterial phagosomes were attenuated in RAW264.7 cells after glucocorticoids treatment. Consequently, we observed a glucocorticoid-induced increase in the survival of intracellular mycobacteria in both primary macrophages and cell lines. Glucocorticoids treatment decreased the activation of TBK1 kinase, which promotes the maturation of autophagosomes. Inhibition of TBK1 also decreased the production of nitric oxide. Furthermore, several autophagy-related genes were down-regulated, while activation of the Akt/mTOR signaling pathway was increased after glucocorticoids treatment, which may account for autophagy inhibition during mycobacterial infection. Restoration of autophagy with the agonist rapamycin abolished glucocorticoid-mediated enhancement of mycobacterial survival, suggesting that glucocorticoids blocked anti-mycobacterial defense via autophagy inhibition. Collectively, this study demonstrates that glucocorticoids impair innate antimicrobial autophagy and promote mycobacterial survival in macrophages, which is a novel mechanism for glucocorticoid-mediated immunosuppression. Our findings may provide important clues for tuberculosis prevention.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- World Health Organization. Global tuberculosis control 2015. World Health Organization (2015).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

