Protein Quality Control by Molecular Chaperones in Neurodegeneration
- PMID: 28428740
- PMCID: PMC5382173
- DOI: 10.3389/fnins.2017.00185
Protein Quality Control by Molecular Chaperones in Neurodegeneration
Abstract
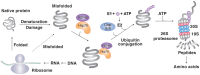
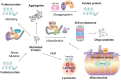
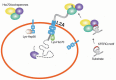
Protein homeostasis (proteostasis) requires the timely degradation of misfolded proteins and their aggregates by protein quality control (PQC), of which molecular chaperones are an essential component. Compared with other cell types, PQC in neurons is particularly challenging because they have a unique cellular structure with long extensions. Making it worse, neurons are postmitotic, i.e., cannot dilute toxic substances by division, and, thus, are highly sensitive to misfolded proteins, especially as they age. Failure in PQC is often associated with neurodegenerative diseases, such as Huntington's disease (HD), Alzheimer's disease (AD), Parkinson's disease (PD), and prion disease. In fact, many neurodegenerative diseases are considered to be protein misfolding disorders. To prevent the accumulation of disease-causing aggregates, neurons utilize a repertoire of chaperones that recognize misfolded proteins through exposed hydrophobic surfaces and assist their refolding. If such an effort fails, chaperones can facilitate the degradation of terminally misfolded proteins through either the ubiquitin (Ub)-proteasome system (UPS) or the autophagy-lysosome system (hereafter autophagy). If soluble, the substrates associated with chaperones, such as Hsp70, are ubiquitinated by Ub ligases and degraded through the proteasome complex. Some misfolded proteins carrying the KFERQ motif are recognized by the chaperone Hsc70 and delivered to the lysosomal lumen through a process called, chaperone-mediated autophagy (CMA). Aggregation-prone misfolded proteins that remain unprocessed are directed to macroautophagy in which cargoes are collected by adaptors, such as p62/SQSTM-1/Sequestosome-1, and delivered to the autophagosome for lysosomal degradation. The aggregates that have survived all these refolding/degradative processes can still be directly dissolved, i.e., disaggregated by chaperones. Studies have shown that molecular chaperones alleviate the pathogenic symptoms by neurodegeneration-causing protein aggregates. Chaperone-inducing drugs and anti-aggregation drugs are actively exploited for beneficial effects on symptoms of disease. Here, we discuss how chaperones protect misfolded proteins from aggregation and mediate the degradation of terminally misfolded proteins in collaboration with cellular degradative machinery. The topics also include therapeutic approaches to improve the expression and turnover of molecular chaperones and to develop anti-aggregation drugs.
Keywords: autophagy-lysosome system; chaperon-mediated autophagy; macroautophagy; protein aggregation; protein quality control; proteolysis; ubiquitin-proteasome system.
Figures
References
-
- Agarraberes F. A., Dice J. F. (2001). A molecular chaperone complex at the lysosomal membrane is required for protein translocation. J. Cell Sci. 114(Pt 13), 2491–2499. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

