Mutational Analysis at Intersubunit Interfaces of an Anionic Glutamate Receptor Reveals a Key Interaction Important for Channel Gating by Ivermectin
- PMID: 28428744
- PMCID: PMC5382172
- DOI: 10.3389/fnmol.2017.00092
Mutational Analysis at Intersubunit Interfaces of an Anionic Glutamate Receptor Reveals a Key Interaction Important for Channel Gating by Ivermectin
Abstract
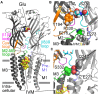
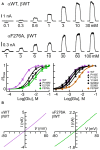
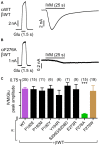
The broad-spectrum anthelmintic drug ivermectin (IVM) activates and stabilizes an open-channel conformation of invertebrate chloride-selective glutamate receptors (GluClRs), thereby causing a continuous inflow of chloride ions and sustained membrane hyperpolarization. These effects suppress nervous impulses and vital physiological processes in parasitic nematodes. The GluClRs are pentamers. Homopentameric receptors assembled from the Caenorhabditis elegans (C. elegans) GluClα (GLC-1) subunit can inherently respond to IVM but not to glutamate (the neurotransmitter). In contrast, heteromeric GluClα/β (GLC-1/GLC-2) assemblies respond to both ligands, independently of each other. Glutamate and IVM bind at the interface between adjacent subunits, far away from each other; glutamate in the extracellular ligand-binding domain, and IVM in the ion-channel pore periphery. To understand the importance of putative intersubunit contacts located outside the glutamate and IVM binding sites, we introduced mutations at intersubunit interfaces, between these two binding-site types. Then, we determined the effect of these mutations on the activation of the heteromeric mutant receptors by glutamate and IVM. Amongst these mutations, we characterized an α-subunit point mutation located close to the putative IVM-binding pocket, in the extracellular end of the first transmembrane helix (M1). This mutation (αF276A) moderately reduced the sensitivity of the heteromeric GluClαF276A/βWT receptor to glutamate, and slightly decreased the receptor subunits' cooperativity in response to glutamate. In contrast, the αF276A mutation drastically reduced the sensitivity of the receptor to IVM and significantly increased the receptor subunits' cooperativity in response to IVM. We suggest that this mutation reduces the efficacy of channel gating, and impairs the integrity of the IVM-binding pocket, likely by disrupting important interactions between the tip of M1 and the M2-M3 loop of an adjacent subunit. We hypothesize that this physical contact between M1 and the M2-M3 loop tunes the relative orientation of the ion-channel transmembrane helices M1, M2 and M3 to optimize pore opening. Interestingly, pre-exposure of the GluClαF276A/βWT mutant receptor to subthreshold IVM concentration recovered the receptor sensitivity to glutamate. We infer that IVM likely retained its positive modulation activity by constraining the transmembrane helices in a preopen orientation sensitive to glutamate, with no need for the aforementioned disrupted interactions between M1 and the M2-M3 loop.
Keywords: Cys-loop receptors; GluCls; ivermectin; ligand-gated ion channels; parasitic nematodes.
Figures



Similar articles
-
Subunit stoichiometry and arrangement in a heteromeric glutamate-gated chloride channel.Proc Natl Acad Sci U S A. 2016 Feb 2;113(5):E644-53. doi: 10.1073/pnas.1423753113. Epub 2016 Jan 20. Proc Natl Acad Sci U S A. 2016. PMID: 26792524 Free PMC article.
-
Trapping of ivermectin by a pentameric ligand-gated ion channel upon open-to-closed isomerization.Sci Rep. 2017 Feb 20;7:42481. doi: 10.1038/srep42481. Sci Rep. 2017. PMID: 28218274 Free PMC article.
-
The neural γ2α1β2α1β2 gamma amino butyric acid ion channel receptor: structural analysis of the effects of the ivermectin molecule and disulfide bridges.J Mol Model. 2018 Jul 14;24(8):206. doi: 10.1007/s00894-018-3739-z. J Mol Model. 2018. PMID: 30008086
-
Emerging issues of connexin channels: biophysics fills the gap.Q Rev Biophys. 2001 Aug;34(3):325-472. doi: 10.1017/s0033583501003705. Q Rev Biophys. 2001. PMID: 11838236 Review.
-
Gating mechanisms in Cys-loop receptors.Eur Biophys J. 2009 Dec;39(1):37-49. doi: 10.1007/s00249-009-0452-y. Epub 2009 Apr 29. Eur Biophys J. 2009. PMID: 19404635 Review.
Cited by
-
Effects of glutamate and ivermectin on single glutamate-gated chloride channels of the parasitic nematode H. contortus.PLoS Pathog. 2017 Oct 2;13(10):e1006663. doi: 10.1371/journal.ppat.1006663. eCollection 2017 Oct. PLoS Pathog. 2017. PMID: 28968469 Free PMC article.
-
Residues in the 1st Transmembrane-Spanning Helix Are Important for GABAAρ Receptor Function.Biomolecules. 2022 Sep 7;12(9):1251. doi: 10.3390/biom12091251. Biomolecules. 2022. PMID: 36139090 Free PMC article.
References
-
- Arevalo E., Chiara D. C., Forman S. A., Cohen J. B., Miller K. W. (2005). Gating-enhanced accessibility of hydrophobic sites within the transmembrane region of the nicotinic acetylcholine receptor’s δ-subunit. A time-resolved photolabeling study. J. Biol. Chem. 280, 13631–13640. 10.1074/jbc.M413911200 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

