Decreased Levels of Foldase and Chaperone Proteins Are Associated with an Early-Onset Amyotrophic Lateral Sclerosis
- PMID: 28428745
- PMCID: PMC5382314
- DOI: 10.3389/fnmol.2017.00099
Decreased Levels of Foldase and Chaperone Proteins Are Associated with an Early-Onset Amyotrophic Lateral Sclerosis
Abstract
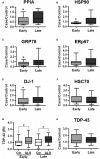
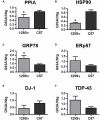
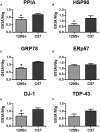
Amyotrophic lateral sclerosis (ALS) is a fatal neurodegenerative disease characterized by a progressive upper and lower motor neuron degeneration. One of the peculiar clinical characteristics of ALS is the wide distribution in age of onset, which is probably caused by different combinations of intrinsic and exogenous factors. We investigated whether these modifying factors are converging into common pathogenic pathways leading either to an early or a late disease onset. This would imply the identification of phenotypic biomarkers, that can distinguish the two populations of ALS patients, and of relevant pathways to consider in a therapeutic intervention. Toward this aim a differential proteomic analysis was performed in peripheral blood mononuclear cells (PBMC) from a group of 16 ALS patients with an age of onset ≤55 years and a group of 16 ALS patients with an age of onset ≥75 years, and matched healthy controls. We identified 43 differentially expressed proteins in the two groups of patients. Gene ontology analysis revealed that there was a significant enrichment in annotations associated with protein folding and response to stress. We next validated a selected number of proteins belonging to this functional group in 85 patients and 83 age- and sex-matched healthy controls using immunoassays. The results of the validation study confirmed that there was a decreased level of peptidyl-prolyl cis-trans isomerase A (also known as cyclophilin A), heat shock protein HSP 90-alpha, 78 kDa glucose-regulated protein (also known as BiP) and protein deglycase DJ-1 in PBMC of ALS patients with an early onset. Similar results were obtained in PBMC and spinal cord from two SOD1G93A mouse models with an early and late disease onset. This study suggests that a different ability to upregulate proteins involved in proteostasis, such as foldase and chaperone proteins, may be at the basis of a different susceptibility to ALS, putting forward the development of therapeutic approaches aiming at boosting the protein quality control system.
Keywords: biomarkers; chaperone; foldase; protein folding; response to stress.
Figures

Similar articles
-
Diagnostic and prognostic values of PBMC proteins in amyotrophic lateral sclerosis.Neurobiol Dis. 2020 Jun;139:104815. doi: 10.1016/j.nbd.2020.104815. Epub 2020 Feb 20. Neurobiol Dis. 2020. PMID: 32087285
-
Guanabenz delays the onset of disease symptoms, extends lifespan, improves motor performance and attenuates motor neuron loss in the SOD1 G93A mouse model of amyotrophic lateral sclerosis.Neuroscience. 2014 Sep 26;277:132-8. doi: 10.1016/j.neuroscience.2014.03.047. Epub 2014 Mar 31. Neuroscience. 2014. PMID: 24699224
-
Targeting Extracellular Cyclophilin A Reduces Neuroinflammation and Extends Survival in a Mouse Model of Amyotrophic Lateral Sclerosis.J Neurosci. 2017 Feb 8;37(6):1413-1427. doi: 10.1523/JNEUROSCI.2462-16.2016. Epub 2016 Dec 23. J Neurosci. 2017. PMID: 28011744 Free PMC article.
-
[Development of motor neuron restorative therapy in amyotrophic lateral sclerosis using hepatocyte growth factor].Rinsho Shinkeigaku. 2009 Nov;49(11):814-7. doi: 10.5692/clinicalneurol.49.814. Rinsho Shinkeigaku. 2009. PMID: 20030218 Review. Japanese.
-
Protein misfolding in the late-onset neurodegenerative diseases: common themes and the unique case of amyotrophic lateral sclerosis.Proteins. 2013 Aug;81(8):1285-303. doi: 10.1002/prot.24285. Epub 2013 Jul 2. Proteins. 2013. PMID: 23508986 Review.
Cited by
-
Effect of RNS60 in amyotrophic lateral sclerosis: a phase II multicentre, randomized, double-blind, placebo-controlled trial.Eur J Neurol. 2023 Jan;30(1):69-86. doi: 10.1111/ene.15573. Epub 2022 Oct 7. Eur J Neurol. 2023. PMID: 36148821 Free PMC article. Clinical Trial.
-
Defective cyclophilin A induces TDP-43 proteinopathy: implications for amyotrophic lateral sclerosis and frontotemporal dementia.Brain. 2021 Dec 31;144(12):3710-3726. doi: 10.1093/brain/awab333. Brain. 2021. PMID: 34972208 Free PMC article.
-
The regulatory role of endoplasmic reticulum chaperone proteins in neurodevelopment.Front Neurosci. 2022 Nov 15;16:1032607. doi: 10.3389/fnins.2022.1032607. eCollection 2022. Front Neurosci. 2022. PMID: 36458041 Free PMC article. Review.
-
Connecting the Dots in the Neuroglobin-Protein Interaction Network of an Unstressed and Ferroptotic Cell Death Neuroblastoma Model.Cells. 2019 Aug 11;8(8):873. doi: 10.3390/cells8080873. Cells. 2019. PMID: 31405213 Free PMC article.
-
Decoding distinctive features of plasma extracellular vesicles in amyotrophic lateral sclerosis.Mol Neurodegener. 2021 Aug 10;16(1):52. doi: 10.1186/s13024-021-00470-3. Mol Neurodegener. 2021. PMID: 34376243 Free PMC article.
References
-
- Batelli S., Albani D., Rametta R., Polito L., Prato F., Pesaresi M., et al. (2008). DJ-1 modulates alpha-synuclein aggregation state in a cellular model of oxidative stress: relevance for Parkinson’s disease and involvement of HSP70. PLoS ONE 3:e1884 10.1371/journal.pone.0001884 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

