Desensitizing Mitochondrial Permeability Transition by ERK-Cyclophilin D Axis Contributes to the Neuroprotective Effect of Gallic Acid against Cerebral Ischemia/Reperfusion Injury
- PMID: 28428752
- PMCID: PMC5382198
- DOI: 10.3389/fphar.2017.00184
Desensitizing Mitochondrial Permeability Transition by ERK-Cyclophilin D Axis Contributes to the Neuroprotective Effect of Gallic Acid against Cerebral Ischemia/Reperfusion Injury
Abstract
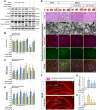
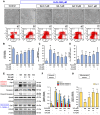
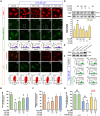
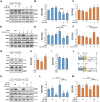
Ischemic stroke is a devastating disease with complex pathophysiology. Much evidence confirms that opening of the mitochondrial permeability transition pore (MPTP) is related with mitochondrial dysfunction to apoptosis in ischemic stroke, thus elucidating its signaling mechanism and screening novel MPTP inhibitor is therefore of paramount importance. Our earlier studies identified that gallic acid (GA), a naturally occurring plant phenol, endows with effect on inhibition of mitochondrial dysfunction, which has significant neuroprotective effect in cerebral ischemia/reperfusion injury. However, its molecular mechanisms regulating mitochondrial dysfunction remain elusive. Here, we uncover a role of GA in protecting mitochondria via MPTP inhibition. In addition to inhibit CypD binding to adenine nucleotide translocator, GA potentiates extracellular signal-regulated kinases (ERK) phosphorylation, leading to a decrease in cyclophilin D (CypD) expression, resulting in a desensitization to induction of MPTP, thus inhibiting caspase activation and ultimately giving rise to cellular survival. Our study firstly identifies ERK-CypD axis is one of the cornerstones of the cell death pathways following ischemic stroke, and confirms GA is a novel inhibitor of MPTP, which inhibits apoptosis depending on regulating the ERK-CypD axis.
Keywords: cerebral ischemia/reperfusion; cyclophilin D (CypD); extracellular signal-regulated kinases (ERK); gallic acid (GA); mitochondrial permeability transition pore (MPTP).
Figures


References
-
- Baumgartner H. K., Gerasimenko J. V., Thorne C., Ferdek P., Pozzan T., Tepikin A. V., et al. (2009). Calcium elevation in mitochondria is the main Ca2+ requirement for mitochondrial permeability transition pore (mPTP) opening. J. Biol. Chem. 284 20796–20803. 10.1074/jbc.M109.025353 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

