Challenge of Humans with Wild-type Salmonella enterica Serovar Typhi Elicits Changes in the Activation and Homing Characteristics of Mucosal-Associated Invariant T Cells
- PMID: 28428786
- PMCID: PMC5382150
- DOI: 10.3389/fimmu.2017.00398
Challenge of Humans with Wild-type Salmonella enterica Serovar Typhi Elicits Changes in the Activation and Homing Characteristics of Mucosal-Associated Invariant T Cells
Abstract
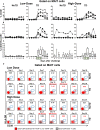
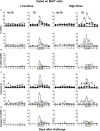
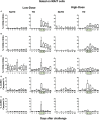
Gastrointestinal infections by Salmonella enterica serovar Typhi (S. Typhi) are rare in industrialized countries. However, they remain a major public health problem in the developing world with an estimated 26.9 million new cases annually and significant mortality when untreated. Recently, we provided the first direct evidence that CD8+ MAIT cells are activated and have the potential to kill cells exposed to S. Typhi, and that these responses are dependent on bacterial load. However, MAIT cell kinetics and function during bacterial infections in humans remain largely unknown. In this study, we characterize the human CD8+ MAIT cell immune response to S. Typhi infection in subjects participating in a challenge clinical trial who received a low- or high dose of wild-type S. Typhi. We define the kinetics of CD8+ MAIT cells as well as their levels of activation, proliferation, exhaustion/apoptosis, and homing potential. Regardless of the dose, in volunteers resistant to infection (NoTD), the levels of CD8+ MAIT cells after S. Typhi challenge fluctuated around their baseline values (day 0). In contrast, volunteers susceptible to the development of typhoid disease (TD) exhibited a sharp decline in circulating MAIT cells during the development of typhoid fever. Interestingly, MAIT cells from low-dose TD volunteers had higher levels of CD38 coexpressing CCR9, CCR6, and Ki67 during the development of typhoid fever than high-dose TD volunteers. No substantial perturbations on the levels of these markers were observed in NoTD volunteers irrespective of the dose. In sum, we describe, for the first time, that exposure to an enteric bacterium, in this case S. Typhi, results in changes in MAIT cell activation, proliferation, and homing characteristics, suggesting that MAIT cells are an important component of the human host response to bacterial infection.
Keywords: MAIT cells; Salmonella; T cells; bacteria; gut; human.
Figures



Similar articles
-
Mucosal-associated invariant T (MAIT) cell responses in Salmonella enterica serovar Typhi strain Ty21a oral vaccine recipients.Oxf Open Immunol. 2025 Mar 25;6(1):iqaf002. doi: 10.1093/oxfimm/iqaf002. eCollection 2025. Oxf Open Immunol. 2025. PMID: 40224569 Free PMC article.
-
Oral Wild-Type Salmonella Typhi Challenge Induces Activation of Circulating Monocytes and Dendritic Cells in Individuals Who Develop Typhoid Disease.PLoS Negl Trop Dis. 2015 Jun 11;9(6):e0003837. doi: 10.1371/journal.pntd.0003837. eCollection 2015 Jun. PLoS Negl Trop Dis. 2015. PMID: 26065687 Free PMC article.
-
Oral Challenge with Wild-Type Salmonella Typhi Induces Distinct Changes in B Cell Subsets in Individuals Who Develop Typhoid Disease.PLoS Negl Trop Dis. 2016 Jun 14;10(6):e0004766. doi: 10.1371/journal.pntd.0004766. eCollection 2016 Jun. PLoS Negl Trop Dis. 2016. PMID: 27300136 Free PMC article.
-
Complex adaptive immunity to enteric fevers in humans: lessons learned and the path forward.Front Immunol. 2014 Oct 27;5:516. doi: 10.3389/fimmu.2014.00516. eCollection 2014. Front Immunol. 2014. PMID: 25386175 Free PMC article. Review.
-
Controlled human infectious models, a path forward in uncovering immunological correlates of protection: Lessons from enteric fevers studies.Front Microbiol. 2022 Sep 20;13:983403. doi: 10.3389/fmicb.2022.983403. eCollection 2022. Front Microbiol. 2022. PMID: 36204615 Free PMC article. Review.
Cited by
-
Contribution of APCs to mucosal-associated invariant T cell activation in infectious disease and cancer.Innate Immun. 2018 May;24(4):192-202. doi: 10.1177/1753425918768695. Epub 2018 Apr 9. Innate Immun. 2018. PMID: 29631470 Free PMC article. Review.
-
Mucosal-associated invariant T cells and disease.Nat Rev Immunol. 2019 Oct;19(10):643-657. doi: 10.1038/s41577-019-0191-y. Nat Rev Immunol. 2019. PMID: 31308521 Review.
-
Mucosal-Associated Invariant T cells exhibit distinct functional signatures associated with protection against typhoid fever.Cell Immunol. 2022 Aug;378:104572. doi: 10.1016/j.cellimm.2022.104572. Epub 2022 Jun 20. Cell Immunol. 2022. PMID: 35772315 Free PMC article.
-
Colonic mucosal associated invariant T cells in Crohn's disease have a diverse and non-public T cell receptor beta chain repertoire.PLoS One. 2023 Nov 3;18(11):e0285918. doi: 10.1371/journal.pone.0285918. eCollection 2023. PLoS One. 2023. PMID: 37922286 Free PMC article.
-
Francisella tularensis induces Th1 like MAIT cells conferring protection against systemic and local infection.Nat Commun. 2021 Jul 16;12(1):4355. doi: 10.1038/s41467-021-24570-2. Nat Commun. 2021. PMID: 34272362 Free PMC article.
References
-
- Tilloy F, Treiner E, Park SH, Garcia C, Lemonnier F, de la Salle H, et al. An invariant T cell receptor alpha chain defines a novel TAP-independent major histocompatibility complex class Ib-restricted alpha/beta T cell subpopulation in mammals. J Exp Med (1999) 189:1907–21.10.1084/jem.189.12.1907 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

