Regulation of Th2 responses by different cell types expressing the interleukin-31 receptor
- PMID: 28428802
- PMCID: PMC5392993
- DOI: 10.1186/s13223-017-0194-9
Regulation of Th2 responses by different cell types expressing the interleukin-31 receptor
Abstract
Background: Interleukin-31 (IL-31) is a recently identified cytokine produced by Th2 cells that is involved in the development of atopic dermatitis-induced skin inflammation and pruritus. Its receptor, IL-31RA, is expressed by a number of cell types, including epithelial cells, eosinophils, and activated monocytes and macrophages. To date, however, the regulation of Th2 responses by distinct cell types and tissues expressing IL-31RA has not been well studied.
Methods: In this study, Cry j 2, one of the major allergens of Japanese cedar pollen, was administered to IL-31RA-deficient or wild-type (WT) mice via nasal or intraperitoneal injection for induction of specific Th2 responses.
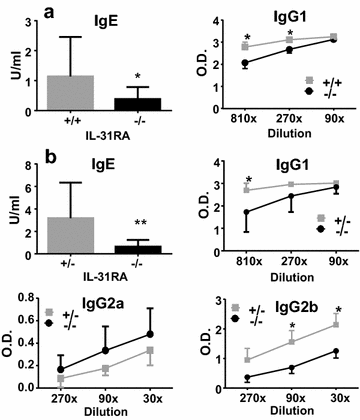
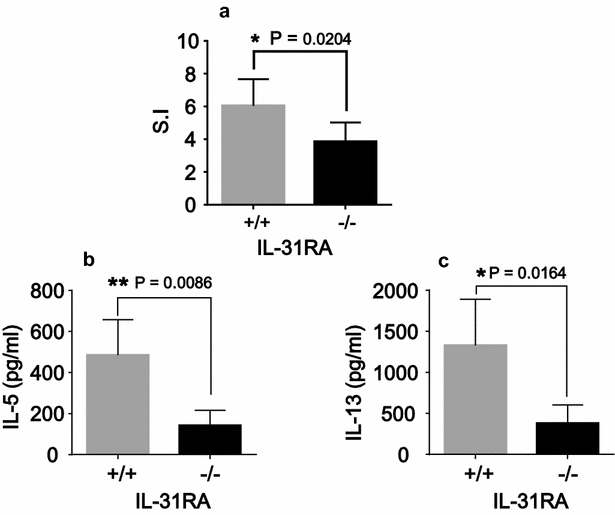
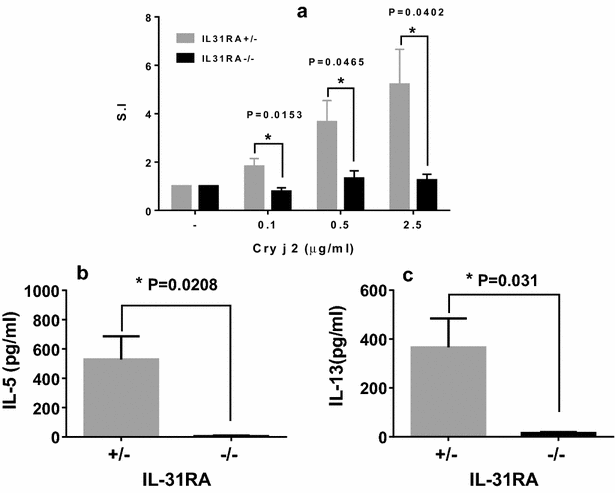
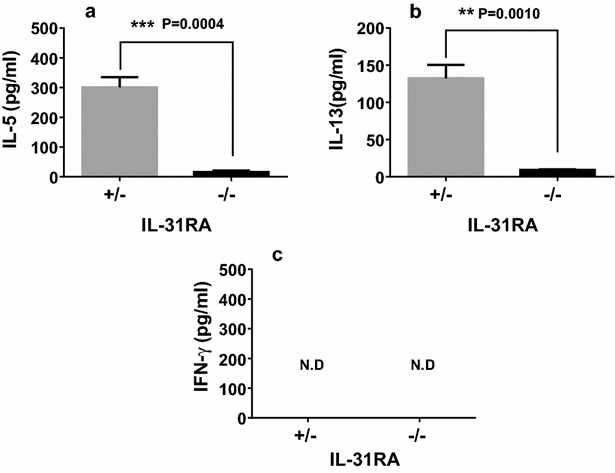
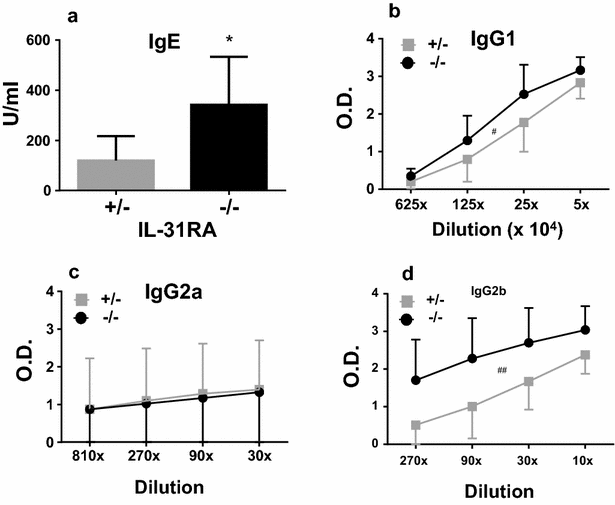
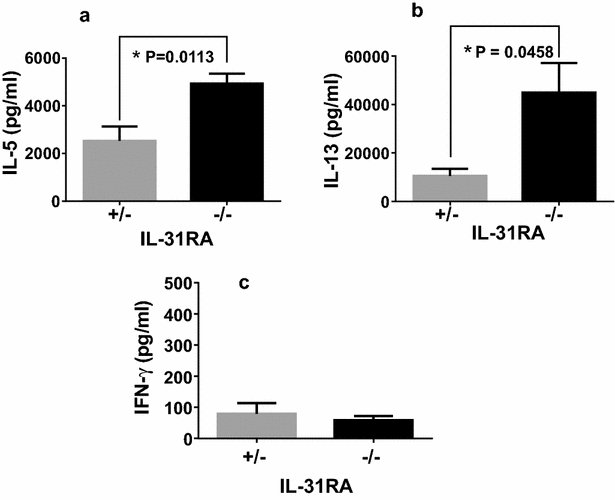
Results: After nasal administration of Cry j 2, IL-31RA-deficient mice showed lower Cry j 2-specific CD4+ T cell proliferation, Th2 cytokine (IL-5 and IL-13) production, and Th2-mediated (IgE, IgG1, and IgG2b) antibody responses than WT mice. In contrast, IL-31RA-deficient mice administered Cry j 2 intraperitoneally showed stronger Th2 immune responses than WT mice.
Conclusions: These results indicate that IL-31R signaling positively regulates Th2 responses induced by nasal administration of Cry j 2, but negatively regulates these responses when Cry j 2 is administered intraperitoneally. Collectively, these data indicate that the induction of antigen-specific Th2 immune responses might depend on tissue-specific cell types expressing IL-31RA.
Keywords: Cry j 2; Deficient mice; IL-31 receptor; IgE; Th2.
Figures
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

