Leucine-rich-repeat-containing variable lymphocyte receptors as modules to target plant-expressed proteins
- PMID: 28428809
- PMCID: PMC5395774
- DOI: 10.1186/s13007-017-0180-8
Leucine-rich-repeat-containing variable lymphocyte receptors as modules to target plant-expressed proteins
Abstract
Background: The ability to target and manipulate protein-based cellular processes would accelerate plant research; yet, the technology to specifically and selectively target plant-expressed proteins is still in its infancy. Leucine-rich repeats (LRRs) are ubiquitously present protein domains involved in mediating protein-protein interactions. LRRs confer the binding specificity to the highly diverse variable lymphocyte receptor (VLR) antibodies (including VLRA, VLRB and VLRC types) that jawless vertebrates make as the functional equivalents of jawed vertebrate immunoglobulin-based antibodies.
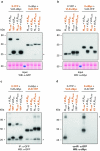
Results: In this study, VLRBs targeting an effector protein from a plant pathogen, HopM1, were developed by immunizing lampreys and using yeast surface display to select for high-affinity VLRBs. HopM1-specific VLRBs (VLRM1) were expressed in planta in the cytosol, the trans-Golgi network, and the apoplast. Expression of VLRM1 was higher when the protein localized to an oxidizing environment that would favor disulfide bridge formation (when VLRM1 was not localized to the cytoplasm), as disulfide bonds are necessary for proper VLR folding. VLRM1 specifically interacted in planta with HopM1 but not with an unrelated bacterial effector protein while HopM1 failed to interact with a non-specific VLRB.
Conclusions: In the future, VLRs may be used as flexible modules to bind proteins or carbohydrates of interest in planta, with broad possibilities for their use by binding directly to their targets and inhibiting their action, or by creating chimeric proteins with new specificities in which endogenous LRR domains are replaced by those present in VLRs.
Keywords: HopM1; Leucine-rich repeat; Modules; Protein targeting; Variable lymphocyte receptor.
Figures
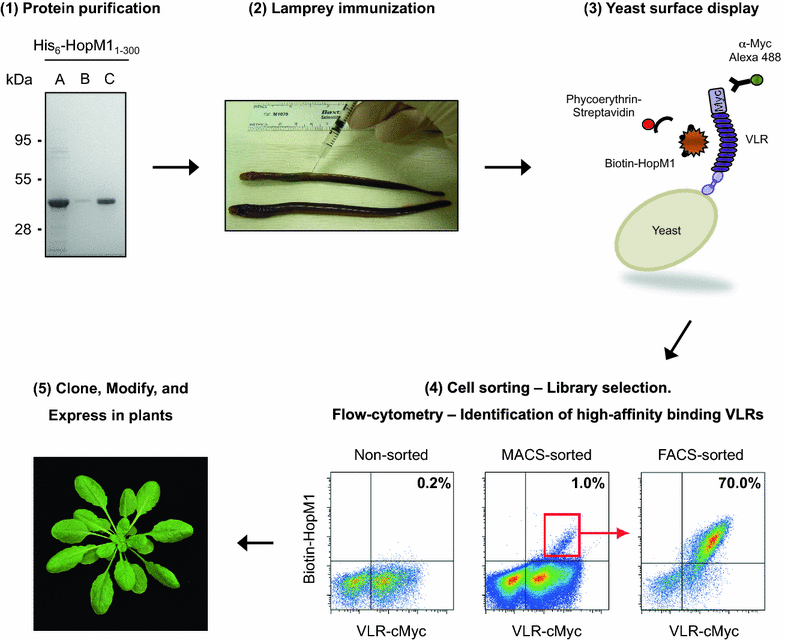
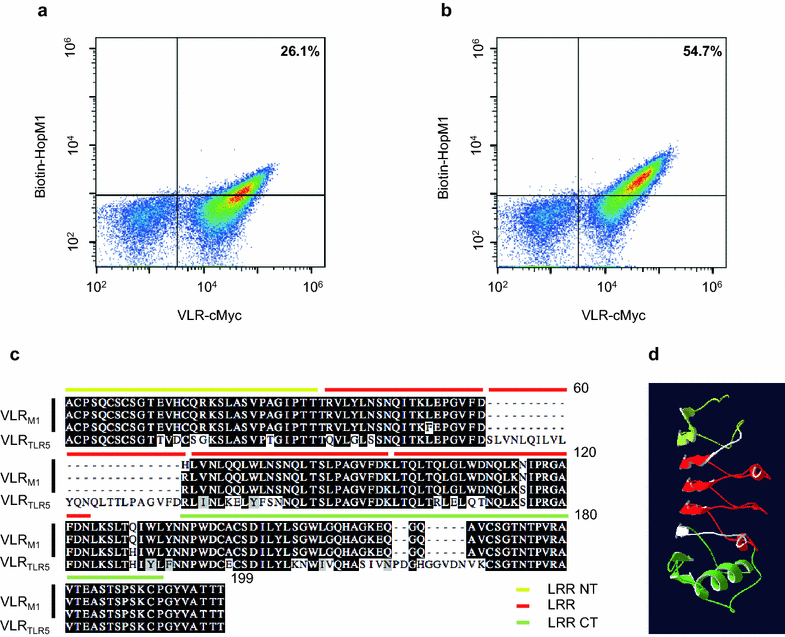
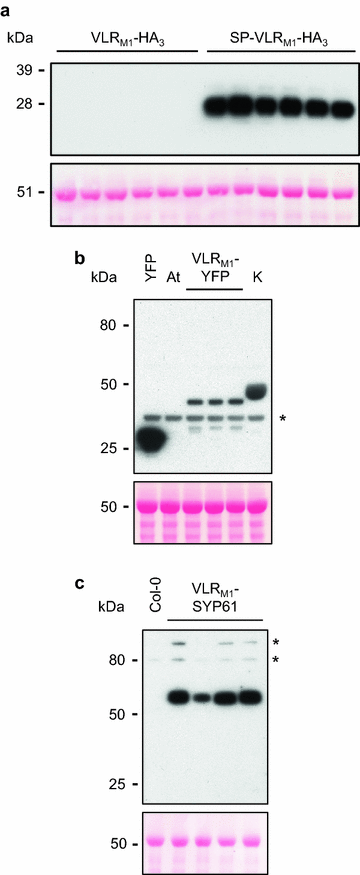
References
-
- Stergiopoulos I, van den Burg HA, Okmen B, Beenen HG, van Liere S, Kema GH, et al. Tomato Cf resistance proteins mediate recognition of cognate homologous effectors from fungi pathogenic on dicots and monocots. Proc Natl Acad Sci USA. 2010;107:7610–7615. doi: 10.1073/pnas.1002910107. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

