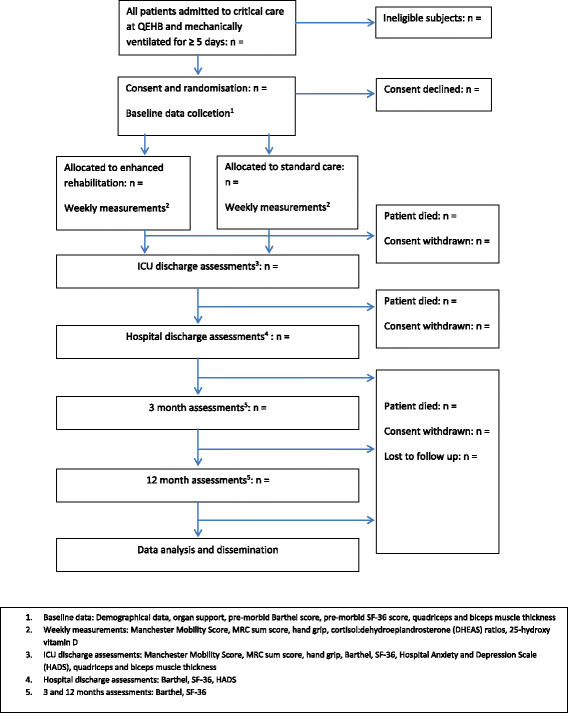
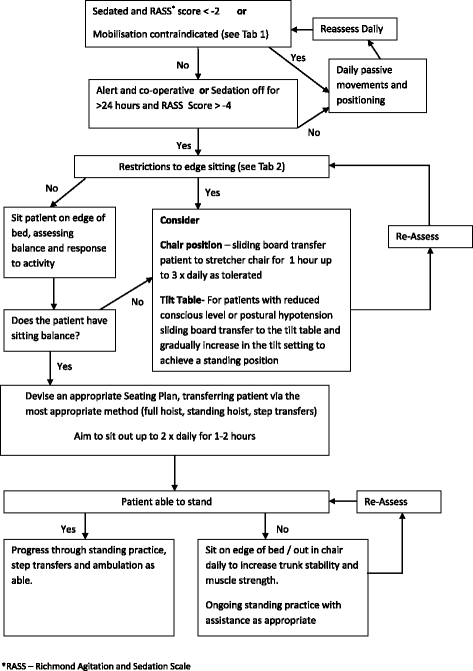
A comparison of earlier and enhanced rehabilitation of mechanically ventilated patients in critical care compared to standard care (REHAB): study protocol for a single-site randomised controlled feasibility trial
- PMID: 28428892
- PMCID: PMC5393007
- DOI: 10.1186/s40814-017-0131-1
A comparison of earlier and enhanced rehabilitation of mechanically ventilated patients in critical care compared to standard care (REHAB): study protocol for a single-site randomised controlled feasibility trial
Abstract
Background: Mortality from critical illness is improving, but survivors suffer from prolonged weakness and psychological and cognitive impairments. Maximising the recovery after critical illness has been highlighted as a research priority, especially in relation to an ageing population who present with higher rates of pre-morbid disability. Small studies have shown that starting rehabilitation early within the intensive care unit (ICU) improves short-term outcomes. Systematic reviews have highlighted the need for robust multicentre randomised controlled trials with longer term follow-up.
Methods: The study design is a randomised controlled study to explore the feasibility of providing earlier and enhanced rehabilitation to mechanically ventilated patients at high risk of ICU-acquired weakness within the ICU. The rehabilitation intervention involves a structured programme, with progression along a functionally based mobility protocol according to set safety criteria. The overall aim of the intervention is to commence mobilisation at an earlier time point in the patient's illness and increase mobility of the patient through their recovery trajectory. Participants will be randomised to enhanced rehabilitation or standard care, with the aim of recruiting at least 100 patients over 16 months. The trial design will assess recruitment and consent rates from eligible patients, compliance with the intervention, and assess a range of possible outcome measures for use in a definitive trial, with follow-up continuing for 12 months post hospital discharge.
Discussion: This study will evaluate the feasibility of providing an earlier and enhanced rehabilitation intervention to mechanically ventilated patients in critical care. We will identify strengths and weaknesses of the proposed protocol and the utility and characteristics of the outcome measures. The results from this study will inform the design of a phase III multicentre trial of enhanced rehabilitation for critically ill adults.
Trial registration: ISRCTN90103222, 13/08/2015; retrospectively registered.
Keywords: Critical care; Mechanical ventilation; Physiotherapy; Rehabilitation.
Figures
Similar articles
-
Earlier and enhanced rehabilitation of mechanically ventilated patients in critical care: A feasibility randomised controlled trial.J Crit Care. 2018 Apr;44:407-412. doi: 10.1016/j.jcrc.2018.01.001. Epub 2018 Jan 4. J Crit Care. 2018. PMID: 29331668 Clinical Trial.
-
Therapeutic respiratory and functional rehabilitation protocol for intensive care unit patients affected by COVID-19: a structured summary of a study protocol for a randomised controlled trial.Trials. 2021 Apr 12;22(1):268. doi: 10.1186/s13063-021-05210-y. Trials. 2021. PMID: 33845878 Free PMC article.
-
A comparison of standard occupational therapy versus early enhanced occupation-based therapy in a medical/surgical intensive care unit: study protocol for a single site feasibility trial (EFFORT-ICU).Pilot Feasibility Stud. 2021 Feb 18;7(1):51. doi: 10.1186/s40814-021-00795-2. Pilot Feasibility Stud. 2021. PMID: 33602337 Free PMC article.
-
Effects of early, combined endurance and resistance training in mechanically ventilated, critically ill patients: a study protocol for a randomised controlled trial.Trials. 2016 Aug 15;17:403. doi: 10.1186/s13063-016-1533-8. Trials. 2016. PMID: 27527501 Free PMC article. Clinical Trial.
-
Protocol-directed sedation versus non-protocol-directed sedation in mechanically ventilated intensive care adults and children.Cochrane Database Syst Rev. 2018 Nov 12;11(11):CD009771. doi: 10.1002/14651858.CD009771.pub3. Cochrane Database Syst Rev. 2018. PMID: 30480753 Free PMC article.
Cited by
-
Short-Term Clinical and Quality Outcomes Have Inconsistent Changes From a Quality Improvement Initiative to Increase Access to Physical Therapy in the Cardiovascular and Surgical ICU.Crit Care Explor. 2019 Oct 30;1(10):e0055. doi: 10.1097/CCE.0000000000000055. eCollection 2019 Oct. Crit Care Explor. 2019. PMID: 32166236 Free PMC article.
-
Short-term effects of mobilization on oxygenation in patients after open surgery for pancreatic cancer: a randomized controlled trial.BMC Surg. 2021 Apr 7;21(1):185. doi: 10.1186/s12893-021-01187-2. BMC Surg. 2021. PMID: 33827537 Free PMC article. Clinical Trial.
References
-
- Griffiths J, Hatch RA, Bishop J, Morgan K, Jenkinson C, Cuthbertson BH, Brett SJ. An exploration of social and economic outcome and associated health-related quality of life after critical illness in general intensive care unit survivors: a 12-month follow-up study. Crit Care. 2013;17(3):R100. doi: 10.1186/cc12745. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources