α-Mangostin decreases β-amyloid peptides production via modulation of amyloidogenic pathway
- PMID: 28429536
- PMCID: PMC6492755
- DOI: 10.1111/cns.12699
α-Mangostin decreases β-amyloid peptides production via modulation of amyloidogenic pathway
Abstract
Aims: β-amyloid (Aβ) aggregation and deposition play a central role in the pathogenic process of Alzheimer's disease (AD). α-Mangostin (α-M), a polyphenolic xanthone, have been shown to dissociate Aβ oligomers. In this study, we further investigated the effect of α-M on Aβ production and its molecular mechanism.
Methods: The Aβ and soluble amyloid precursor protein α (sAPPα) in culture medium of cortical neurons were measured by ELISA. The activities of α-, β-, and γ-secretases were assayed, and the interaction between α-M and β- or γ-secretases was simulated by molecular docking.
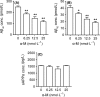
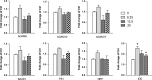
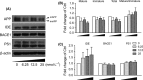
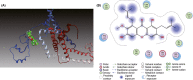
Results: α-M significantly decreased Aβ40 and Aβ42 production. α-M did not affect the expression of enzymes involved in nonamyloidogenic and amyloidogenic pathways, but significantly decreased the activities of β-secretase and likely γ-secretase with IC50 13.22 nmol·L-1 and 16.98 nmol·L-1 , respectively. Molecular docking demonstrated that α-M interacted with β-site amyloid precursor protein cleaving enzyme 1 and presenilin 1 to interfere with their active sites.
Conclusions: Our data demonstrate that α-M decreases Aβ production through inhibiting activities of β-secretase and likely γ-secretase in the amyloidogenic pathway. The current data together with previous study indicated that α-M could be a novel neuroprotective agent through intervention of multiple pathological processes of AD.
Keywords: Alzheimer's disease; α-mangostin; β-amyloid; β-secretase; γ-secretase.
© 2017 John Wiley & Sons Ltd.
Conflict of interest statement
The authors have no conflict of interest.
Figures


Similar articles
-
Auraptene increases the production of amyloid-β via c-Jun N-terminal kinase-dependent activation of γ-secretase.J Alzheimers Dis. 2015;43(4):1215-28. doi: 10.3233/JAD-141692. J Alzheimers Dis. 2015. PMID: 25147119
-
Α-mangostin, a polyphenolic xanthone derivative from mangosteen, attenuates β-amyloid oligomers-induced neurotoxicity by inhibiting amyloid aggregation.Neuropharmacology. 2012 Feb;62(2):871-81. doi: 10.1016/j.neuropharm.2011.09.016. Epub 2011 Sep 24. Neuropharmacology. 2012. PMID: 21958557
-
Cryptotanshinione upregulates alpha-secretase by activation PI3K pathway in cortical neurons.Brain Res. 2010 Aug 12;1348:165-73. doi: 10.1016/j.brainres.2010.05.083. Epub 2010 Jun 2. Brain Res. 2010. PMID: 20595051
-
Genes and mechanisms involved in beta-amyloid generation and Alzheimer's disease.Eur Arch Psychiatry Clin Neurosci. 1999;249(6):266-70. doi: 10.1007/s004060050098. Eur Arch Psychiatry Clin Neurosci. 1999. PMID: 10653281 Review.
-
Disease-modifying therapies in Alzheimer's disease: how far have we come?Drugs. 2006;66(16):2075-93. doi: 10.2165/00003495-200666160-00004. Drugs. 2006. PMID: 17112302 Review.
Cited by
-
Mangosteen Pericarp and Its Bioactive Xanthones: Potential Therapeutic Value in Alzheimer's Disease, Parkinson's Disease, and Depression with Pharmacokinetic and Safety Profiles.Int J Mol Sci. 2020 Aug 27;21(17):6211. doi: 10.3390/ijms21176211. Int J Mol Sci. 2020. PMID: 32867357 Free PMC article. Review.
-
Lessons from Exploring Chemical Space and Chemical Diversity of Propolis Components.Int J Mol Sci. 2020 Jul 15;21(14):4988. doi: 10.3390/ijms21144988. Int J Mol Sci. 2020. PMID: 32679731 Free PMC article. Review.
-
Multifunctional Anti-Alzheimer's Disease Effects of Natural Xanthone Derivatives: A Primary Structure-Activity Evaluation.Front Chem. 2022 May 11;10:842208. doi: 10.3389/fchem.2022.842208. eCollection 2022. Front Chem. 2022. PMID: 35646819 Free PMC article.
-
Acetylcholinesterase Inhibitors in the Treatment of Neurodegenerative Diseases and the Role of Acetylcholinesterase in their Pathogenesis.Int J Mol Sci. 2021 Aug 27;22(17):9290. doi: 10.3390/ijms22179290. Int J Mol Sci. 2021. PMID: 34502198 Free PMC article. Review.
-
Synergetic therapy of glioma mediated by a dual delivery system loading α-mangostin and doxorubicin through cell cycle arrest and apoptotic pathways.Cell Death Dis. 2020 Oct 28;11(10):928. doi: 10.1038/s41419-020-03133-1. Cell Death Dis. 2020. PMID: 33116114 Free PMC article.
References
-
- Awasthi M, Singh S, Pandey VP, Dwivedi UN. Alzheimer's disease: an overview of amyloid beta dependent pathogenesis and its therapeutic implications along with in silico approaches emphasizing the role of natural products. J Neurol Sci. 2016;361:256‐271. - PubMed
-
- Daulatzai MA. Pharmacotherapy and Alzheimer's disease: the M‐drugs (melatonin, minocycline, modafinil, and memantine) approach. Curr Pharm Des. 2016;22:2411‐2430. - PubMed
-
- Mangialasche F, Solomon A, Winblad B, Mecocci P, Kivipelto M. Alzheimer's disease: clinical trials and drug development. Lancet Neurol. 2010;9:702‐716. - PubMed
-
- Ballard C, Gauthier S, Corbett A, Brayne C, Aarsland D, Jones E. Alzheimer's disease. Lancet. 2011;377:1019‐1031. - PubMed
-
- Bullock R. Efficacy and safety of memantine in moderate‐to‐severe Alzheimer disease: the evidence to date. Alzheimer Dis Assoc Disord. 2006;20:23‐29. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

