Intra-arterial verapamil post-thrombectomy is feasible, safe, and neuroprotective in stroke
- PMID: 28429604
- PMCID: PMC5669346
- DOI: 10.1177/0271678X17705259
Intra-arterial verapamil post-thrombectomy is feasible, safe, and neuroprotective in stroke
Abstract
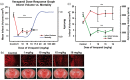
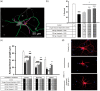
Large vessel ischemic stroke represents the most disabling subtype. While t-PA and endovascular thrombectomy can recanalize the occluded vessel, good clinical outcomes are not uniformly achieved. We propose that supplementing endovascular thrombectomy with superselective intra-arterial (IA) verapamil immediately following recanalization could be safe and effective. Verapamil, a calcium channel blocker, has been shown to be an effective IA adjunct in a pre-clinical mouse focal ischemia model. To demonstrate translational efficacy, mechanism, feasibility, and safety, we conducted a group of translational experiments. We performed in vivo IA dose-response evaluation in our animal stroke model with C57/Bl6 mice. We evaluated neuroprotective mechanism through in vitro primary cortical neuron (PCN) cultures. Finally, we performed a Phase I trial, SAVER-I, to evaluate feasibility and safety of administration in the human condition. IA verapamil has a likely plateau or inverted-U dose-response with a defined toxicity level in mice (LD50 16-17.5 mg/kg). Verapamil significantly prevented PCN death and deleterious ischemic effects. Finally, the SAVER-I clinical trial showed no evidence that IA verapamil increased the risk of intracranial hemorrhage or other adverse effect/procedural complication in human subjects. We conclude that superselective IA verapamil administration immediately following thrombectomy is safe and feasible, and has direct, dose-response-related benefits in ischemia.
Keywords: Ischemic stroke; emergent large vessel occlusion; neuroprotection; thrombectomy; verapamil.
Figures



References
-
- Saver JL, Goyal M, Bonafe A, et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. New Engl J Med 2015; 372: 2285–2295. - PubMed
-
- Bretz MN, Graves A, West A, et al. Steps against recurrent stroke plus: patient transition program. J Neurosci Nurs: J Am Assoc Neurosci Nurse 2014; 46: E3–13. quiz E1-E2. - PubMed
-
- Thacker C. Stroke falls to No. 5 cause of death in U.S. American Heart Association 30 December 2014. http://newsroom.heart.org/news/stroke-falls-to-no-5-cause-of-death-in-u-s.
-
- Summers D, Leonard A, Wentworth D, et al. Comprehensive overview of nursing and interdisciplinary care of the acute ischemic stroke patient: a scientific statement from the American Heart Association. Stroke; a journal of cerebral circulation 2009; 40: 2911–44. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

