Cell Biology of Hyphal Growth
- PMID: 28429675
- PMCID: PMC11687463
- DOI: 10.1128/microbiolspec.FUNK-0034-2016
Cell Biology of Hyphal Growth
Abstract
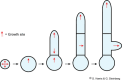
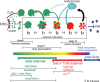
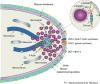
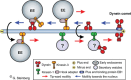
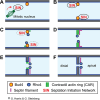
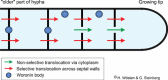
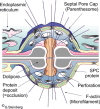
Filamentous fungi are a large and ancient clade of microorganisms that occupy a broad range of ecological niches. The success of filamentous fungi is largely due to their elongate hypha, a chain of cells, separated from each other by septa. Hyphae grow by polarized exocytosis at the apex, which allows the fungus to overcome long distances and invade many substrates, including soils and host tissues. Hyphal tip growth is initiated by establishment of a growth site and the subsequent maintenance of the growth axis, with transport of growth supplies, including membranes and proteins, delivered by motors along the cytoskeleton to the hyphal apex. Among the enzymes delivered are cell wall synthases that are exocytosed for local synthesis of the extracellular cell wall. Exocytosis is opposed by endocytic uptake of soluble and membrane-bound material into the cell. The first intracellular compartment in the endocytic pathway is the early endosomes, which emerge to perform essential additional functions as spatial organizers of the hyphal cell. Individual compartments within septated hyphae can communicate with each other via septal pores, which allow passage of cytoplasm or organelles to help differentiation within the mycelium. This article introduces the reader to more detailed aspects of hyphal growth in fungi.
Figures


References
-
- Evans CS, Hedger JN. 2001. Degradation of plant cell wall polymers, p 1–26. In Gadd GM (ed), Fungi in Bioremediation. Cambridge University Press, Cambridge, United Kingdom. 10.1017/CBO9780511541780.002 - DOI
-
- Brundrett MC. 2009. Mycorrhizal associations and other means of nutrition of vascular plants: understanding the global diversity of host plants by resolving conflicting information and developing reliable means of diagnosis. Plant Soil 320:37–77. 10.1007/s11104-008-9877-9 - DOI
Publication types
MeSH terms
Substances
Grants and funding
- BB/G009872/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/G00465X/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/F022956/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/J009903/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/H019774/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

