Estrogen reprograms the activity of neutrophils to foster protumoral microenvironment during mammary involution
- PMID: 28429725
- PMCID: PMC5399373
- DOI: 10.1038/srep46485
Estrogen reprograms the activity of neutrophils to foster protumoral microenvironment during mammary involution
Abstract
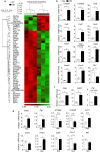
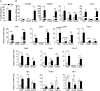
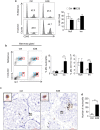
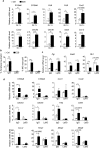
Epidemiological studies have indicated increased risk for breast cancer within 10 years of childbirth. Acute inflammation during mammary involution has been suggested to promote this parity-associated breast cancer. We report here that estrogen exacerbates mammary inflammation during involution. Microarray analysis shows that estrogen induces an extensive proinflammatory gene signature in the involuting mammary tissue. This is associated with estrogen-induced neutrophil infiltration. Furthermore, estrogen induces the expression of protumoral cytokines/chemokines, COX-2 and tissue-remodeling enzymes in isolated mammary neutrophils and systemic neutrophil depletion abolished estrogen-induced expression of these genes in mammary tissue. More interestingly, neutrophil depletion diminished estrogen-induced growth of ERα-negative mammary tumor 4T1 in Balb/c mice. These findings highlight a novel aspect of estrogen action that reprograms the activity of neutrophils to create a pro-tumoral microenvironment during mammary involution. This effect on the microenvironment would conceivably aggravate its known neoplastic effect on mammary epithelial cells.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

