Verteporfin-induced formation of protein cross-linked oligomers and high molecular weight complexes is mediated by light and leads to cell toxicity
- PMID: 28429726
- PMCID: PMC5399488
- DOI: 10.1038/srep46581
Verteporfin-induced formation of protein cross-linked oligomers and high molecular weight complexes is mediated by light and leads to cell toxicity
Retraction in
-
Retraction Note: Verteporfin-induced formation of protein cross-linked oligomers and high molecular weight complexes is mediated by light and leads to cell toxicity.Sci Rep. 2024 May 14;14(1):11040. doi: 10.1038/s41598-024-61893-8. Sci Rep. 2024. PMID: 38744880 Free PMC article. No abstract available.
Abstract
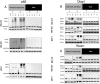
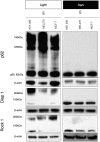
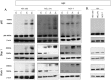
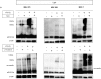
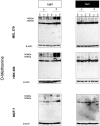
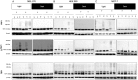
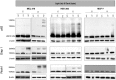
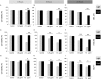
Verteporfin (VP) was first used in Photodynamic therapy, where a non-thermal laser light (689 nm) in the presence of oxygen activates the drug to produce highly reactive oxygen radicals, resulting in local cell and tissue damage. However, it has also been shown that Verteporfin can have non-photoactivated effects such as interference with the YAP-TEAD complex of the HIPPO pathway, resulting in growth inhibition of several neoplasias. More recently, it was proposed that, another non-light mediated effect of VP is the formation of cross-linked oligomers and high molecular weight protein complexes (HMWC) that are hypothesized to interfere with autophagy and cell growth. Here, in a series of experiments, using human uveal melanoma cells (MEL 270), human embryonic kidney cells (HEK) and breast cancer cells (MCF7) we showed that Verteporfin-induced HMWC require the presence of light. Furthermore, we showed that the mechanism of this cross-linking, which involves both singlet oxygen and radical generation, can occur very efficiently even after lysis of the cells, if the lysate is not protected from ambient light. This work offers a better understanding regarding VP's mechanisms of action and suggests caution when one studies the non-light mediated actions of this drug.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
Similar articles
-
Verteporfin inhibits growth of human glioma in vitro without light activation.Sci Rep. 2017 Aug 8;7(1):7602. doi: 10.1038/s41598-017-07632-8. Sci Rep. 2017. PMID: 28790340 Free PMC article.
-
Determination of the migration effect and molecular docking of verteporfin in different subtypes of breast cancer cells.Mol Med Rep. 2020 Nov;22(5):3955-3961. doi: 10.3892/mmr.2020.11482. Epub 2020 Sep 2. Mol Med Rep. 2020. PMID: 32901856 Free PMC article.
-
Verteporfin inhibits cell proliferation and induces apoptosis in different subtypes of breast cancer cell lines without light activation.BMC Cancer. 2020 Oct 29;20(1):1042. doi: 10.1186/s12885-020-07555-0. BMC Cancer. 2020. PMID: 33121449 Free PMC article.
-
The Hippo Pathway and YAP/TAZ-TEAD Protein-Protein Interaction as Targets for Regenerative Medicine and Cancer Treatment.J Med Chem. 2015 Jun 25;58(12):4857-73. doi: 10.1021/jm501615v. Epub 2015 Mar 11. J Med Chem. 2015. PMID: 25719868 Review.
-
YAP/TAZ for cancer therapy: opportunities and challenges (review).Int J Oncol. 2015 Apr;46(4):1444-52. doi: 10.3892/ijo.2015.2877. Epub 2015 Feb 5. Int J Oncol. 2015. PMID: 25652178 Review.
Cited by
-
KRAS as a Key Oncogene in the Clinical Precision Diagnosis and Treatment of Pancreatic Cancer.J Cancer. 2022 Aug 31;13(11):3209-3220. doi: 10.7150/jca.76695. eCollection 2022. J Cancer. 2022. PMID: 36118526 Free PMC article. Review.
-
Verteporfin-Loaded Mesoporous Silica Nanoparticles' Topical Applications Inhibit Mouse Melanoma Lymphangiogenesis and Micrometastasis In Vivo.Int J Mol Sci. 2021 Dec 14;22(24):13443. doi: 10.3390/ijms222413443. Int J Mol Sci. 2021. PMID: 34948239 Free PMC article.
-
Mechanistic Insights into Photodynamic Regulation of Adenosine 5'-Triphosphate-Binding Cassette Drug Transporters.ACS Pharmacol Transl Sci. 2021 Sep 15;4(5):1578-1587. doi: 10.1021/acsptsci.1c00138. eCollection 2021 Oct 8. ACS Pharmacol Transl Sci. 2021. PMID: 36118950 Free PMC article.
-
Verteporfin-induced lysosomal compartment dysregulation potentiates the effect of sorafenib in hepatocellular carcinoma.Cell Death Dis. 2019 Oct 3;10(10):749. doi: 10.1038/s41419-019-1989-z. Cell Death Dis. 2019. PMID: 31582741 Free PMC article.
-
Characterization of Perturbing Actions by Verteporfin, a Benzoporphyrin Photosensitizer, on Membrane Ionic Currents.Front Chem. 2019 Aug 22;7:566. doi: 10.3389/fchem.2019.00566. eCollection 2019. Front Chem. 2019. PMID: 31508407 Free PMC article.
References
-
- Husain D. et al.. Effects of photodynamic therapy using verteporfin on experimental choroidal neovascularization and normal retina and choroid up to 7 weeks after treatment. Invest Ophthalmol Vis Sci 40, 2322–2331 (1999). - PubMed
-
- Kessel D. In vitro photosensitization with a benzoporphyrin derivative. Photochem Photobiol 49, 579–582 (1989). - PubMed
-
- Kramer M. et al.. Liposomal benzoporphyrin derivative verteporfin photodynamic therapy. Selective treatment of choroidal neovascularization in monkeys. Ophthalmology 103, 427–438 (1996). - PubMed
-
- Miller J. W. et al.. Photodynamic therapy with verteporfin for choroidal neovascularization caused by age-related macular degeneration: results of a single treatment in a phase 1 and 2 study. Arch Ophthalmol 117, 1161–1173 (1999). - PubMed
-
- Renno R. Z. & Miller J. W. Photosensitizer delivery for photodynamic therapy of choroidal neovascularization. Adv Drug Deliv Rev 52, 63–78 (2001). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

